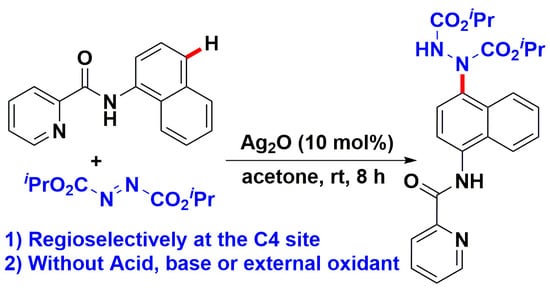
Silver(I)-Catalyzed C4-H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates at Room Temperature
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure for Synthesis of Product 3aa
3.3. General Procedure for Synthesis of Product 3
3.4. Spectral Data for Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Lygaitis, R.; Getautis, V.; Grazulevicius, J.V. Hole-Transporting Hydrazones. Chem. Soc. Rev. 2008, 37, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chen, J. Arylamine Organic Dyes for Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Guram, A.S.; Rennels, R.A.; Buchwald, S.L. A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines. Angew. Chem. Int. Ed. Engl. 1995, 34, 1348–1350. [Google Scholar] [CrossRef]
- Louie, J.; Hartwig, J.F. Palladium-Catalyzed Synthesis of Arylamines from Aryl Halides. Mechanistic Studies Lead to Coupling in the Absence of Tin Reagents. Tetrahedron Lett. 1995, 36, 3609–3612. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, C.H.; Jiao, N. Recent Advances in Copper-Catalyzed Dehydrogenative Functionalization via a Single Electron Transfer (SET) Process. Chem. Soc. Rev. 2012, 41, 3464–3484. [Google Scholar] [CrossRef]
- Ping, L.; Chung, D.S.; Bouffard, J.; Lee, S.G. Transition Metal-Catalyzed Site- and Regio-Divergent C-H Bond Functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328. [Google Scholar] [CrossRef]
- Yang, L.; Huang, H.M. Transition-Metal-Catalyzed Direct Addition of Unactivated C-H Bonds to Polar Unsaturated Bonds. Chem. Rev. 2015, 115, 3468–3517. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, P.; Zhang, M.; Su, W.P. Metal-Catalyzed Decarboxylative C-H Functionalization. Chem. Rev. 2017, 117, 8864–8907. [Google Scholar] [CrossRef]
- Rej, S.; Ano, Y.; Chatani, N. Bidentate Directing Groups: An Efficient Tool in C-H Bond Functionalization Chemistry for the Expedient Construction of C-C Bonds. Chem. Rev. 2020, 120, 1788–1887. [Google Scholar] [CrossRef]
- Large, B.; Prim, D. C-H Functionalization Strategies in the Naphthalene Series: Site Selections and Functional Diversity. Synthesis 2020, 52, 2600–2612. [Google Scholar]
- Prevost, S. Regioselective C-H Functionalization of Naphthalenes: Reactivity and Mechanistic Insights. ChemPlusChem 2020, 85, 476–486. [Google Scholar] [CrossRef]
- Zaitsev, V.G.; Shabashov, D.; Daugulis, O. Highly Regioselective Arylation of sp3 C-H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. [Google Scholar] [CrossRef]
- Xiao, Q.; Meng, X.T.; Kanai, M.; Kuninobu, Y. Palladium-Catalyzed C-H Fluorosilylation of 2-Phenylpyridines: Synthesis of Silafluorene Equivalents. Angew. Chem. Int. Ed. 2014, 53, 3168–3172. [Google Scholar] [CrossRef]
- Xu, C.F.; Shen, Q.L. Palladium-Catalyzed Trifluoromethylthiolation of Aryl C-H Bonds. Org. Lett. 2014, 16, 2046–2049. [Google Scholar] [CrossRef]
- Patel, P.; Chang, S. N-Substituted Hydroxylamines as Synthetically Versatile Amino Sources in the Iridium-Catalyzed Mild C-H Amidation Reaction. Org. Lett. 2014, 16, 3328–3331. [Google Scholar] [CrossRef]
- Iwasaki, M.; Iyanaga, M.; Tsuchiya, Y.; Nishimura, Y.; Li, W.J.; Li, Z.P.; Nishihara, Y. Palladium-Catalyzed Direct Thiolation of Aryl C-H Bonds with Disulfides. Chem. Eur. J. 2014, 20, 2459–2462. [Google Scholar] [CrossRef]
- Zhang, X.; Si, W.L.; Bao, M.; Asao, N.; Yamamoto, Y.; Jin, T.N. Rh(III)-Catalyzed Regioselective Functionalization of C-H Bonds of Naphthylcarbamates for Oxidative Annulation with Alkynes. Org. Lett. 2014, 16, 4830–4833. [Google Scholar] [CrossRef]
- Kondrashov, M.; Raman, S.; Wendt, O.F. Metal Controlled Regioselectivity in the Cyclometallation of 2-(1-Naphthyl)Pyridine. Chem. Commun. 2015, 51, 911–913. [Google Scholar] [CrossRef]
- Yang, Q.L.; Wang, X.Y.; Lu, J.Y.; Zhang, L.P.; Fang, P.; Mei, T.S. Copper-Catalyzed Electrochemical C-H Amination of Arenes with Secondary Amines. J. Am. Chem. Soc. 2018, 140, 11487–11494. [Google Scholar] [CrossRef]
- Begam, H.M.; Choudhury, R.; Behera, A.; Jana, R. Copper-Catalyzed Electrophilic ortho C(sp2)-H Amination of Aryl Amines: Dramatic Reactivity of Bicyclic System. Org. Lett. 2019, 21, 4651–4656. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed Directed C(sp2)-H and C(sp3)-H Functionalization with Trimethylaluminum. J. Am. Chem. Soc. 2015, 137, 7660–7663. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.H.; Han, L.; Wang, L.L.; Song, H.; Chu, W.Y.; Sun, Z.Z. Direct Cyanation of Picolinamides Using K4[Fe(CN)6] as the Cyanide Source. Chem. Lett. 2015, 44, 743–745. [Google Scholar] [CrossRef]
- Li, Z.X.; Sun, S.Y.; Qiao, H.J.; Yang, F.; Zhu, Y.; Kang, J.X.; Wu, Y.S.; Wu, Y.J. Palladium-Catalyzed Regioselective C8-H Amination of 1-Naphthylamine Derivatives with Aliphatic Amines. Org. Lett. 2016, 18, 4594–4597. [Google Scholar] [CrossRef]
- Lan, J.Y.; Xie, H.S.; Lu, X.X.; Deng, Y.F.; Jiang, H.F.; Zeng, W. Co(II)-Catalyzed Regioselective Cross-Dehydrogenative Coupling of Aryl C-H Bonds with Carboxylic Acids. Org. Lett. 2017, 19, 4279–4282. [Google Scholar] [CrossRef]
- Rej, S.; Chatani, N. Rhodium(I)-Catalyzed C8-Alkylation of 1-Naphthylamide Derivatives with Alkenes through a Bidentate Picolinamide Chelation System. ACS Catal. 2018, 8, 6699–6706. [Google Scholar] [CrossRef]
- Xiong, Y.S.; Yu, Y.; Weng, J.; Lu, G. Copper-Catalyzed Peri-Selective Direct Sulfenylation of 1-Naphthylamines with Disulfides. Org. Chem. Front. 2018, 5, 982–989. [Google Scholar] [CrossRef]
- Roy, S.; Pradhan, S.; Punniyamurthy, T. Copper-Mediated Regioselective C-H Etherification of Naphthylamides with Arylboronic Acids Using Water as an Oxygen Source. Chem. Commun. 2018, 54, 3899–3902. [Google Scholar] [CrossRef]
- Yu, X.M.; Yang, F.; Wu, Y.S.; Wu, Y.J. Palladium-Catalyzed C8-H Acylation of 1-Naphthylamines with Acyl Chlorides. Org. Lett. 2019, 21, 1726–1729. [Google Scholar] [CrossRef]
- Li, J.M.; Wang, Y.H.; Yu, Y.; Wu, R.B.; Weng, J.; Lu, G. Copper-Catalyzed Remote C-H Functionalizations of Naphthyla-mides through a Coordinating Activation Strategy and Single-Electron-Transfer (SET) Mechanism. ACS Catal. 2017, 7, 2661–2667. [Google Scholar] [CrossRef]
- Bai, P.R.; Sun, S.Y.; Li, Z.X.; Qiao, H.J.; Su, X.X.; Yang, F.; Wu, Y.S.; Wu, Y.J. Ru/Cu Photoredox or Cu/Ag Catalyzed C4-H Sulfonylation of 1-Naphthylamides at Room Temperature. J. Org. Chem. 2017, 82, 12119–12127. [Google Scholar] [CrossRef]
- Liang, S.; Bolte, M.; Mano-likakes, G. Copper-Catalyzed Remote para-C-H Functionalization of Anilines with Sodium and Lithium Sulfinates. Chem. Eur. J. 2017, 23, 96–100. [Google Scholar] [CrossRef]
- Zhu, H.M.; Sun, S.Y.; Qiao, H.J.; Yang, F.; Kang, J.X.; Wu, Y.S.; Wu, Y.J. Silver(I)-Catalyzed C4-H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates. Org. Lett. 2018, 20, 620–623. [Google Scholar] [CrossRef]
- You, G.R.; Wang, K.; Wang, X.D.; Wang, G.D.; Sun, J.; Duan, G.Y.; Xia, C.C. Visible-Light-Mediated Nickel(II)-Catalyzed C-N Cross-Coupling in Water: Green and Regioselective Access for the Synthesis of Pyrazole-Containing Compounds. Org. Lett. 2018, 20, 4005–4009. [Google Scholar] [CrossRef]
- Xu, J.; Du, K.; Shen, J.B.; Shen, C.; Chai, K.J.; Zhang, P.F. Copper(II)-Catalyzed Selective Para Amination of Arylamine with Pyrazole by C-H Functionalization. ChemCatChem 2018, 10, 3675–3679. [Google Scholar] [CrossRef]
- Pei, M.X.; Zu, C.H.; Liu, Z.; Yang, F.; Wu, Y.J. Merging Photoredox Catalysis with Transition Metal Catalysis: Direct C4-H Sulfamidation of 1 Naphthylamine Derivatives. J. Org. Chem. 2021, 86, 11324–11332. [Google Scholar] [CrossRef]
- Zhao, L.X.; Sun, M.M.; Yang, F.; Wu, Y.J. Silver(I) Promoted the C4–H Bond Phosphonation of 1-Naphthylamine Derivatives with H-Phosphonates. J. Org. Chem. 2021, 86, 11519–11530. [Google Scholar] [CrossRef]
- Sahoo, T.; Sen, C.; Singh, H.; Suresh, E.; Ghosh, S.C. Copper-Catalyzed C4 Carboxylation of 1-Naphthylamide Derivatives with CBr4/MeOH. Adv. Synth. Catal. 2019, 361, 3950–3957. [Google Scholar] [CrossRef]
- Kumar, S.; Pradhan, S.; Roy, S.; De, P.B.; Punniyamurthy, T. Iron-Catalyzed Regioselective Remote C(sp2)-H Carboxylation of Naphthyl and Quinoline Amides. J. Org. Chem. 2019, 84, 10481–10489. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.B.; Chu, Z.Z.; Xia, C.C. Transition-Metal Free Oxidative C-H Etherification of Acylanilines with Alcohols through a Radical Pathway. Org. Biomol. Chem. 2019, 17, 6346–6350. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.J.; Sun, S.Y.; Yang, F.; Zhu, Y.; Zhu, W.G.; Dong, Y.X.; Wu, Y.S.; Kong, X.T.; Jiang, L.; Wu, Y.J. Copper(I)-Catalyzed Sulfonylation of 8-Aminoquinoline Amides with Sulfonyl Chlorides in Air. Org. Lett. 2015, 17, 6086–6089. [Google Scholar] [CrossRef]
- Sun, M.M.; Sun, S.Y.; Qiao, H.J.; Yang, F.; Zhu, Y.; Kang, J.X.; Wu, Y.S.; Wu, Y.J. Silver(I)-Promoted C5-H Phosphonation of 8-Aminoquinoline Amides with H-Phosphonates. Org. Chem. Front. 2016, 3, 1646–1650. [Google Scholar] [CrossRef]
- Qiao, H.J.; Sun, S.Y.; Yang, F.; Zhu, Y.; Kang, J.X.; Wu, Y.S.; Wu, Y.J. Merging Photoredox Catalysis with Iron(III) Catalysis: C5-H Bromination and Iodination of 8-Aminoquinoline Amides in Water. Adv. Synth. Catal. 2017, 359, 1976–1980. [Google Scholar] [CrossRef]
- Qiao, H.J.; Sun, S.Y.; Zhang, Y.; Zhu, H.M.; Yu, X.M.; Li, Z.X.; Yang, F.; Wu, Y.S.; Wu, Y.J. Merging Photoredox Catalysis with Transition Metal Catalysis: Site-Selective C4 or C5-H Phosphonation of 8-Aminoquinoline Amides. Org. Chem. Front. 2017, 4, 1981–1986. [Google Scholar] [CrossRef]







 | |||
| Entry | Catalyst | Solvent | Yield (%) b |
| 1 | Ag2O | DCE | 80 |
| 2 | Ag2O | dioxane | 90 |
| 3 | Ag2O | acetone | 97 |
| 4 | Ag2O | THF | 90 |
| 5 | Ag2O | DME | 80 |
| 6 | Ag2O | DCM | <5 |
| 7 | CuO | acetone | <5 |
| 8 | Cu2O | acetone | <5 |
| 9 | Fe2O3 | acetone | <5 |
| 10 | AgOAc | acetone | <5 |
| 11 | - | acetone | <5 |
| 12 c | Ag2O | acetone | 88 |
| 13 d | Ag2O | acetone | 46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Pei, M.; Yang, F.; Wu, Y. Silver(I)-Catalyzed C4-H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates at Room Temperature. Catalysts 2022, 12, 1006. https://doi.org/10.3390/catal12091006
Zhang Y, Pei M, Yang F, Wu Y. Silver(I)-Catalyzed C4-H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates at Room Temperature. Catalysts. 2022; 12(9):1006. https://doi.org/10.3390/catal12091006
Chicago/Turabian StyleZhang, Yuxue, Mengxue Pei, Fan Yang, and Yangjie Wu. 2022. "Silver(I)-Catalyzed C4-H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates at Room Temperature" Catalysts 12, no. 9: 1006. https://doi.org/10.3390/catal12091006
APA StyleZhang, Y., Pei, M., Yang, F., & Wu, Y. (2022). Silver(I)-Catalyzed C4-H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates at Room Temperature. Catalysts, 12(9), 1006. https://doi.org/10.3390/catal12091006