Homogeneous and Heterogeneous Catalytic Ozonation of Textile Wastewater: Application and Mechanism
Abstract
:1. Introduction
2. Basic of Ozonation
3. Catalytic Ozonation
- Recovery of homogeneous catalysts is difficult and expensive, while in heterogeneous, it is easy and cheaper.
- Homogeneous shows poor thermal stability compared to heterogeneous.
- During homogeneous catalyst application, the selectivity is very good and focused on a single active site, while in heterogeneous, it is weaker, but more functioning for active centers.
4. Homogeneous Catalytic Ozonation
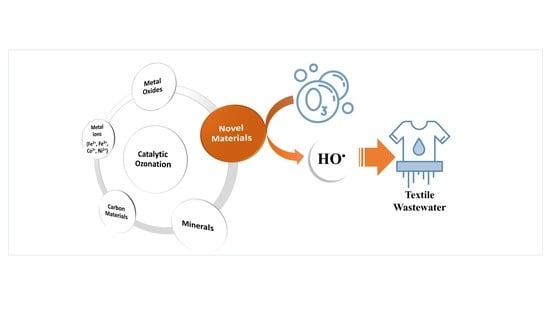
5. Heterogeneous Catalytic Ozonation
5.1. Metal Oxides
5.2. Carbon Materials
5.3. Minerals
5.4. Novel Materials
6. Conclusions
- Increasing the degradation of pollutants in water, mainly organic.
- Supporting the mineralization of organic compounds.
- Reduces ozone consumption compared to the ozonation process itself. When reviewing the parameters used in the studies with the best results, some relationships can be noticed:
- The pH of the solution affects the charge of active centers located on the catalyst surface and the ionic charge of organic molecules. This parameter is responsible for the interaction between the catalyst and the impurities. Low pH slows down the ozone decomposition process, which contributes to the longer contact time of ozone with pollutants, but from the industrial point of view, it is less profitable.
- An important parameter due to the increased interest in environmental protection and costs for the company is the stability of the catalyst and the possibility of its reuse.
- By increasing the amount of the catalyst, we provide more active sites, contributing to the decomposition of ozone, i.e., increasing the reactive radicals in the solution.
- Increasing the ozone flow rate also increases the generation of reactive radicals. This parameter is limited by the number of active sites on the catalyst surface.
- Increasing the ozone dose increases gas permeation into the dye solution/sewage, thus improving its availability to react with pollutants. Increasing the ozone dose is also associated with higher production costs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bisschops, I.; Spanjers, H. Literature Review on Textile Wastewater Characterisation. Environ. Technol. 2008, 24, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V. Production, Characterization and Treatment of Textile Effluents: A Critical Review. J. Chem. Eng. Process Technol. 2013, 5, 1–19. [Google Scholar] [CrossRef]
- McFarlane, D. Wastewater as a Potential Source of Recycling in The-Peel Region; Water Corporation: Perth, Australia, 2019. [Google Scholar]
- Sojka-Ledakowicz, J.; Zylla, R.; Mrozinska, Z.; Pazdzior, K.; Klepacz-Smolka, A.; Ledakowicz, S. Application of Membrane Processes in Closing of Water Cycle in a Textile Dye-House. Desalination 2010, 250, 634–638. [Google Scholar] [CrossRef]
- Hossain, L.; Sarker, S.K.; Khan, M.S. Evaluation of Present and Future Wastewater Impacts of Textile Dyeing Industries in Bangladesh. Environ. Dev. 2018, 26, 23–33. [Google Scholar] [CrossRef]
- Reddy, A.S.; Kalla, S.; Murthy, Z.V.P. Textile Wastewater Treatment via Membrane Distillation. Environ. Eng. Res. 2022, 27, 210228. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Z.; Gu, C.; Shen, C.; Ma, C.; Liu, Y.; Yin, S.; Xu, C. Metals Pollution from Textile Production Wastewater in Chinese Southeastern Coastal Area: Occurrence, Source Identification, and Associated Risk Assessment. Environ. Sci. Pollut. Res. 2021, 28, 38689–38697. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Kanwar, R.; Singh, J. Physicochemical Assessment of Industrial Textile Effluents of Punjab (India). Appl. Water Sci. 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Senthil Kumar, P.; Saravanan, A. Sustainable Wastewater Treatments in Textile Sector; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081020425. [Google Scholar]
- The European Commission, Integrated Pollution Prevention and Control (IPPC). Reference Document on Best Available Techniques for the Textiles Industry; EU Publ.: Brussels, Belgium, 2003. [Google Scholar]
- OECD. OECD Due Diligence Guidance for Responsible Supply Chains in the Garment and Footwear Sector; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Ortega-Egea, J.M.; García-de-Frutos, N. Greenpeace’s Detox Campaign: Towards a More Sustainable Textile Industry; Springer International Publishing: Cham, Switzerland, 2019; pp. 37–47. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A Review of the Existing and Emerging Technologies in the Combination of AOPs and Biological Processes in Industrial Textile Wastewater Treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and Biological Treatments for Wastewater Decontamination-A Review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods. An Updated Review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Ahlström, L.H.; Sparr Eskilsson, C.; Björklund, E. Determination of Banned Azo Dyes in Consumer Goods. TrAC-Trends Anal. Chem. 2005, 24, 49–56. [Google Scholar] [CrossRef]
- European Parliament. Directive 2002/61/EC of the European Parliament and of the Council of 19 July 2002 Amending for the Nineteenth Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Pre; EU Publ.: Brussels, Belgium, 19 July 2002. [Google Scholar]
- Allègre, C.; Moulin, P.; Maisseu, M.; Charbit, F. Treatment and Reuse of Reactive Dyeing Effluents. J. Memb. Sci. 2006, 269, 15–34. [Google Scholar] [CrossRef]
- Cay, A.; Tarakçioǧlu, I.; Hepbasli, A. Assessment of Finishing Processes by Exhaustion Principle for Textile Fabrics: An Exergetic Approach. Appl. Therm. Eng. 2009, 29, 2554–2561. [Google Scholar] [CrossRef]
- Lu, X.; Liu, L.; Liu, R.; Chen, J. Textile Wastewater Reuse as an Alternative Water Source for Dyeing and Finishing Processes: A Case Study. Desalination 2010, 258, 229–232. [Google Scholar] [CrossRef]
- Al-Momani, F.; Touraud, E.; Degorce-Dumas, J.R.; Roussy, J.; Thomas, O. Biodegradability Enhancement of Textile Dyes and Textile Wastewater by VUV Photolysis. J. Photochem. Photobiol. A Chem. 2002, 153, 191–197. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Gao, W. Reviewing Textile Wastewater Produced by Industries: Characteristics, Environmental Impacts, and Treatment Strategies. Water Sci. Technol. 2022, 85, 2076–2096. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef]
- Chung, J.; Kim, J.-O.O. Application of Advanced Oxidation Processes to Remove Refractory Compounds from Dye Wastewater. Desalin. Water Treat. 2011, 25, 233–240. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of Various Advanced Oxidation Processes and Chemical Treatment Methods for COD and Color Removal from a Polyester and Acetate Fiber Dyeing Effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef]
- Emami-Meibodi, M.; Parsaeian, M.R.M.-R.; Banaei, M. Ozonation and Electron Beam Irradiation of Dyes Mixture. Ozone Sci. Eng. 2013, 35, 49–54. [Google Scholar] [CrossRef]
- Hsing, H.J.; Chiang, P.C.; Chang, E.E.; Chen, M.Y. The Decolorization and Mineralization of Acid Orange 6 Azo Dye in Aqueous Solution by Advanced Oxidation Processes: A Comparative Study. J. Hazard. Mater. 2007, 141, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Oguz, E.; Keskinler, B. Comparison among O3, PAC Adsorption, O3/HCO3−, O3/H2O2 and O3/PAC Processes for the Removal of Bomaplex Red CR-L Dye from Aqueous Solution. Dye. Pigment. 2007, 74, 329–334. [Google Scholar] [CrossRef]
- Muthukumar, M.; Sargunamani, D.; Selvakumar, N. Statistical Analysis of the Effect of Aromatic, Azo and Sulphonic Acid Groups on Decolouration of Acid Dye Effluents Using Advanced Oxidation Processes. Dye. Pigment. 2005, 65, 151–158. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioglu, I.A.; Tuhkanen, T. Oxidative Treatment of Simulated Dyehouse Effluent by UV and Near-UV Light Assisted Fenton’s Reagent. Chemosphere 1999, 39, 2767–2783. [Google Scholar] [CrossRef]
- Alaton, I.; Balcioglu, I.A.; Bahnemann, D. Advanced Oxidation of a Reactive Dyebath Effluent:Comparison of O3,H2O2/UV-C and TiO2/UV-A Processes. Water Res. 2002, 36, 1143–1154. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between Industrial and Simulated Textile Wastewater Treatment by AOPs—Biodegradability, Toxicity and Cost Assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, P.H.; Sosamony, K.J. A Comparative Study of Homogeneous and Heterogeneous Photo-Fenton Process for Textile Wastewater Treatment. Procedia Technol. 2016, 24, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Sahunin, C.; Kaewboran, J.; Hunsom, M. Treatment of Textile Dyeing Wastewater by Photo Oxidation Using UV/H2O2/Fe2+ Reagents. ScienceAsia 2006, 32, 181–186. [Google Scholar] [CrossRef]
- Rejman, A. Bioindykacja i Oddziaływanie Na Organizmy Żywe Ścieków Przemysłu Włókienniczego. Probl. Ekol. 2007, 11, 41–46. [Google Scholar]
- Siddique, K.; Rizwan, M.; Shahid, M.J.; Ali, S.; Ahmad, R.; Rizvi, H. Textile Wastewater Treatment Options: A Critical Review; Enhancing Cleanup of Environmental Pollutants; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 183–207. ISBN 978-3-319-55422-8. [Google Scholar]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic Ozonation and Methods of Enhancing Molecular Ozone Reactions in Water Treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Fernando, J. Beltran Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Ikehata, K.; Li, Y. Ozone-Based Processes. Adv. Oxid. Process. Wastewater Treat. Emerg. Green Chem. Technol. 2018, 5, 115–134. [Google Scholar] [CrossRef]
- Biń, A.K. Ozone Dissolution in Aqueous Systems Treatment of the Experimental Data. Exp. Therm. Fluid Sci. 2004, 28, 395–405. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Brine Recycling from Industrial Textilewastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop. Water 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.F.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Bastos, F.C.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Noble Metal–TiO2 Supported Catalysts for the Catalytic Ozonation of Parabens Mixtures. Process Saf. Environ. Prot. 2017, 111, 148–159. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The Efficiency and Mechanisms of Catalytic Ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Nawrocki, J. Catalytic Ozonation in Water: Controversies and Questions. Discussion Paper. Appl. Catal. B Environ. 2013, 142–143, 465–471. [Google Scholar] [CrossRef]
- Legube, B.; Karpel Vel Leitner, N. Catalytic Ozonation: A Promising Advanced Oxidation Technology for Water Treatment. Catal. Today 1999, 53, 61–72. [Google Scholar] [CrossRef]
- Gracia, R.; Aragües, J.L.; Ovelleiro, J.L. Study of the Catalytic Ozonation of Humic Substances in Water and Their Ozonation Byproducts. Ozone Sci. Eng. 1996, 18, 195–208. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Effect of Dissolved Cobalt(II) on the Ozonation of Oxalic Acid. Environ. Sci. Technol. 2002, 36, 4046–4051. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Solid Phase Catalytic Ozonation Process for the Destruction of a Model Pollutant. Ozone Sci. Eng. 2003, 25, 25–39. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, J.; Ma, J.; Qiang, Z. Fluorescence Spectroscopic Characterization of DOM Fractions Isolated from a Filtered River Water after Ozonation and Catalytic Ozonation. Chemosphere 2008, 71, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Bakht Shokouhi, S.; Dehghanzadeh, R.; Aslani, H.; Shahmahdi, N. Activated Carbon Catalyzed Ozonation (ACCO) of Reactive Blue 194 Azo Dye in Aqueous Saline Solution: Experimental Parameters, Kinetic and Analysis of Activated Carbon Properties. J. Water Process Eng. 2020, 35, 101188. [Google Scholar] [CrossRef]
- Bertini, I. Inorganic and Bio-Inorganic Chemistry—Volume II—Homogeneous and Heterogeneous Catalysis; Eolss: Paris, France, 2009; pp. 50–56. [Google Scholar]
- Wang, J.; Chen, H. Catalytic Ozonation for Water and Wastewater Treatment: Recent Advances and Perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Rame, R. Potential of Catalytic Ozonation in Treatment of Industrial Textile Wastewater in Indonesia: Review. J. Ris. Teknol. Pencegah. Pencemaran Ind. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Chang, C.L. Homogeneous Catalytic Ozonation of C.I. Reactive Red 2 by Metallic Ions in a Bubble Column Reactor. J. Hazard. Mater. 2008, 154, 748–755. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Catalytic Ozone Pretreatment of Complex Textile Effluent Using Fe2+ and Zero Valent Iron Nanoparticles. J. Hazard. Mater. 2018, 357, 363–375. [Google Scholar] [CrossRef]
- Vergara-Sánchez, J.; Pérez-Orozco, J.P.; Suárez-Parra, R.; Hernández-Pérez, I. Degradation of Reactive Red 120 Azo Dye in Aqueous Solutions Using Homogeneous/Heterogeneous Iron Systems Degradac on Del Colorante Azo Rojo Reactivo 120 En Soluciones Acuosas Usando Sistemas Homo Eneos/Hetero Eneos De Hierro. Abril Rev. Mex. Ing. Química 2009, 8, 121–131. [Google Scholar]
- Wu, C.H.; Kuo, C.Y.; Chang, C.L. Decolorization of C.I. Reactive Red 2 by Catalytic Ozonation Processes. J. Hazard. Mater. 2008, 153, 1052–1058. [Google Scholar] [CrossRef]
- Trapido, M.; Veressinina, Y.; Munter, R.; Kallas, J. Catalytic Ozonation of M-Dinitrobenzene. Ozone Sci. Eng. 2005, 27, 359–363. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Sanchirico, R. Manganese-Catalysed Ozonation of Glyoxalic Acid in Aqueous Solutions. J. Chem. Technol. Biotechnol. 2000, 75, 59–65. [Google Scholar] [CrossRef]
- Polat, D.; Balci, I.; Özbelge, T.A. Catalytic Ozonation of an Industrial Textile Wastewater in a Heterogeneous Continuous Reactor. J. Environ. Chem. Eng. 2015, 3, 1860–1871. [Google Scholar] [CrossRef]
- Erol, F.; Özbelge, T.A. Catalytic Ozonation with Non-Polar Bonded Alumina Phases for Treatment of Aqueous Dye Solutions in a Semi-Batch Reactor. Chem. Eng. J. 2008, 139, 272–283. [Google Scholar] [CrossRef]
- Qiu, X.H.; Su, X.Y.; Li, X.J.; Li, N. Preparation of CeO2 with Different Morphologies and Its Application to Catalytic Ozonation of Lemon Yellow Solutions. Adv. Mater. Res. 2014, 997, 3–8. [Google Scholar] [CrossRef]
- Chokshi, N.P.; Ruparelia, J.P. Degradation of Reactive Black 5 Azo Dye Using Catalytic Ozonation with MgO. 2016, III, 2–6. [Google Scholar]
- Moussavi, G.; Mahmoudi, M. Degradation and Biodegradability Improvement of the Reactive Red 198 Azo Dye Using Catalytic Ozonation with MgO Nanocrystals. Chem. Eng. J. 2009, 152, 1–7. [Google Scholar] [CrossRef]
- Kong, L.; Diao, Z.; Chang, X.; Xiong, Y.; Chen, D. Synthesis of Recoverable and Reusable Granular MgO-SCCA-Zn Hybrid Ozonation Catalyst for Degradation of Methylene Blue. J. Environ. Chem. Eng. 2016, 4, 4385–4391. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Monteiro, D.C.M.; Órfão, J.J.M.; Pereira, M.F.R. Cerium, Manganese and Cobalt Oxides as Catalysts for the Ozonation of Selected Organic Compounds. Chemosphere 2009, 74, 818–824. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Lo Meo, P.; Aprile, C.; Noto, R. Oxidative Degradation Properties of Co-Based Catalysts in the Presence of Ozone. Appl. Catal. B Environ. 2007, 75, 281–289. [Google Scholar] [CrossRef]
- Chokshi, N.P.; Bhutia, H.; Chotalia, A.; Kadiwala, A.; Ruparelia, J.P. Heterogeneous Catalytic Ozonation of Reactive Black 5 with Cobalt Oxide. Int. J. ChemTech Res. 2017, 10, 402–409. [Google Scholar]
- Li, X.; Chen, W.; Ma, L.; Wang, H.; Fan, J. Industrial Wastewater Advanced Treatment via Catalytic Ozonation with an Fe-Based Catalyst. Chemosphere 2018, 195, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Ma, L.; Huang, Y.; Wang, H. Characteristics and Mechanisms of Catalytic Ozonation with Fe-Shaving-Based Catalyst in Industrial Wastewater Advanced Treatment. J. Clean. Prod. 2019, 222, 174–181. [Google Scholar] [CrossRef]
- Quan, X.; Luo, D.; Wu, J.; Li, R.; Cheng, W.; Ge, S. Ozonation of Acid Red 18 Wastewater Using O3/Ca(OH)2 System in a Micro Bubble Gas-Liquid Reactor. J. Environ. Chem. Eng. 2017, 5, 283–291. [Google Scholar] [CrossRef]
- Wu, G.; Wei, W.; Cui, L. Adsorption and Catalytic Ozonation Performance of Activated Carbon and Cobalt-Supported Activated Carbon Derived from Brewing Yeast. Can. J. Chem. Eng. 2014, 92, 36–40. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, G.Q.; Liu, C.; Zhang, R.N.; Chen, X.X.; Zhang, L. Synergistic Effect of Microbubbles and Activated Carbon on the Ozonation Treatment of Synthetic Dyeing Wastewater. Sep. Purif. Technol. 2018, 201, 10–18. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Bilińska, M.; Gmurek, M. Industrial Textile Wastewater Ozone Treatment: Catalyst Selection. Catalysts 2020, 10, 611. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, H.Y.; Liu, X.; Shi, H.X.; Zhou, T.H.; Wang, C.; Huo, Z.Y.; Wu, Q.Y. Combination of Catalytic Ozonation by Regenerated Granular Activated Carbon (RGAC) and Biological Activated Carbon in the Advanced Treatment of Textile Wastewater for Reclamation. Chemosphere 2019, 231, 369–377. [Google Scholar] [CrossRef]
- Gholami-Borujeni, F.; Naddafi, K.; Nejatzade-Barandozi, F. Application of Catalytic Ozonation in Treatment of Dye from Aquatic Solutions. Desalin. Water Treat. 2013, 51, 6545–6551. [Google Scholar] [CrossRef]
- Gül, Ş.; Özcan, Ö.; Erbatur, O. Ozonation of C.I. Reactive Red 194 and C.I. Reactive Yellow 145 in Aqueous Solution in the Presence of Granular Activated Carbon. Dye. Pigment. 2007, 75, 426–431. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of Aniline Promoted by Activated Carbon. Chemosphere 2007, 67, 809–815. [Google Scholar] [CrossRef]
- Gül, Ş.; Eren, O.; Kir, Ş.; Önal, Y. A Comparison of Different Activated Carbon Performances on Catalytic Ozonation of a Model Azo Reactive Dye. Water Sci. Technol. 2012, 66, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodi, N.M. Photocatalytic Ozonation of Dyes Using Multiwalled Carbon Nanotube. J. Mol. Catal. A Chem. 2013, 366, 254–260. [Google Scholar] [CrossRef]
- Qu, R.; Xu, B.; Meng, L.; Wang, L.; Wang, Z. Ozonation of Indigo Enhanced by Carboxylated Carbon Nanotubes: Performance Optimization, Degradation Products, Reaction Mechanism and Toxicity Evaluation. Water Res. 2015, 68, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Activated Carbon and Ceria Catalysts Applied to the Catalytic Ozonation of Dyes and Textile Effluents. Appl. Catal. B Environ. 2009, 88, 341–350. [Google Scholar] [CrossRef]
- Hu, E.; Shang, S.; Tao, X.M.; Jiang, S.; Chiu, K. lok Regeneration and Reuse of Highly Polluting Textile Dyeing Effluents through Catalytic Ozonation with Carbon Aerogel Catalysts. J. Clean. Prod. 2016, 137, 1055–1065. [Google Scholar] [CrossRef]
- Niu, Z.; Yue, T.; Hu, W.; Sun, W.; Hu, Y.; Xu, Z. Covalent Bonding of MnO2 onto Graphene Aerogel Forwards: Efficiently Catalytic Degradation of Organic Wastewater. Appl. Surf. Sci. 2019, 496, 1–9. [Google Scholar] [CrossRef]
- Cardoso, R.M.F.; Cardoso, I.M.F.; da Silva, L.P.; da Silva, J.C.G.E. Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes. Nanomaterials 2022, 12, 1211. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Mineralisation of Coloured Aqueous Solutions by Ozonation in the Presence of Activated Carbon. Water Res. 2005, 39, 1461–1470. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R.; Omran, N.R. Development of an Efficient Catalyst from Magnetite Ore: Characterization and Catalytic Potential in the Ozonation of Water Toxic Contaminants. Appl. Catal. A Gen. 2012, 445–446, 42–49. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.D.; Roy, R.; Azzouz, A. Acid-Treated Clay Catalysts for Organic Dye Ozonation—Thorough Mineralization through Optimum Catalyst Basicity and Hydrophilic Character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; di Luca, C.; Žerjav, G.; Grau, J.M.; Pintar, A.; Haure, P. Catalytic Ozonation of an Azo-Dye Using a Natural Aluminosilicate. Catal. Today 2021, 361, 24–29. [Google Scholar] [CrossRef]
- Valdés, H.; Godoy, H.P.; Zaror, C.A. Heterogeneous Catalytic Ozonation of Cationic Dyes Using Volcanic Sand. Water Sci. Technol. 2010, 61, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; He, K.; Zhao, B.; Yin, Y.; Yin, L.; Zhang, A. Catalytic Ozonation of Azo Dye Active Brilliant Red X-3B in Water with Natural Mineral Brucite. Catal. Commun. 2007, 8, 1599–1603. [Google Scholar] [CrossRef]
- Valdés, H.; Farfán, V.J.; Manoli, J.A.; Zaror, C.A. Catalytic Ozone Aqueous Decomposition Promoted by Natural Zeolite and Volcanic Sand. J. Hazard. Mater. 2009, 165, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Taseidifar, M.; Khataee, A.; Vahid, B.; Khorram, S.; Joo, S.W. Production of Nanocatalyst from Natural Magnetite by Glow Discharge Plasma for Enhanced Catalytic Ozonation of an Oxazine Dye in Aqueous Solution. J. Mol. Catal. A Chem. 2015, 404–405, 218–226. [Google Scholar] [CrossRef]
- Chokshi, N.P.; Patel, D.; Atkotiya, R.; Ruparelia, J.P. Catalytic Ozonation of Reactive Black 5 in Aqueous Solution over a La-Co-O Catalyst. J. Indian Chem. Soc. 2020, 97, 373–378. [Google Scholar]
- Chokshi, N.P.; Ruparelia, J.P. Synthesis of Nano Ag-La-Co Composite Metal Oxide for Degradation of RB 5 Dye Using Catalytic Ozonation Process. Ozone Sci. Eng. 2021, 44, 182–195. [Google Scholar] [CrossRef]
- Asgari, G.; Faradmal, J.; Nasab, H.Z.; Ehsani, H. Catalytic Ozonation of Industrial Textile Wastewater Using Modified C-Doped MgO Eggshell Membrane Powder. Adv. Powder Technol. 2019, 30, 1297–1311. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Ozonation of Reactive Orange 4 Dye Aqueous Solution Using Mesoporous Cu/SBA-15 Catalytic Material. J. Water Process Eng. 2018, 23, 217–229. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic Ozonation of Dye Industry Effluent Using Mesoporous Bimetallic Ru-Cu/SBA-15 Catalyst. Process Saf. Environ. Prot. 2018, 118, 125–132. [Google Scholar] [CrossRef]
- Zhu, S.N.; Hui, K.S.N.; Hong, X.; Hui, K.S.N. Catalytic Ozonation of Basic Yellow 87 with a Reusable Catalyst Chip. Chem. Eng. J. 2014, 242, 180–186. [Google Scholar] [CrossRef]
- Li, P.; Miao, R.; Wang, P.; Sun, F.; Li, X. yan Bi-Metal Oxide-Modified Flat-Sheet Ceramic Membranes for Catalytic Ozonation of Organic Pollutants in Wastewater Treatment. Chem. Eng. J. 2021, 426, 131263. [Google Scholar] [CrossRef]
- Liang, C.; Luo, X.; Hu, Y. Enhanced Ozone Oxidation by a Novel Fe/Mn@γ−Al2O3 Nanocatalyst: The Role of Hydroxyl Radical and Singlet Oxygen. Water 2022, 14, 19. [Google Scholar] [CrossRef]
- Lu, J.; Wei, X.; Chang, Y.; Tian, S.; Xiong, Y. Role of Mg in Mesoporous MgFe2O4 for Efficient Catalytic Ozonation of Acid Orange II. J. Chem. Technol. Biotechnol. 2016, 91, 985–993. [Google Scholar] [CrossRef]
- El Hassani, K.; Kalnina, D.; Turks, M.; Beakou, B.H.; Anouar, A. Enhanced Degradation of an Azo Dye by Catalytic Ozonation over Ni-Containing Layered Double Hydroxide Nanocatalyst. Sep. Purif. Technol. 2019, 210, 764–774. [Google Scholar] [CrossRef]
- Feng, C.; Diao, P. Nickel Foam Supported NiFe2O4-NiO Hybrid: A Novel 3D Porous Catalyst for Efficient Heterogeneous Catalytic Ozonation of Azo Dye and Nitrobenzene. Appl. Surf. Sci. 2021, 541, 148683. [Google Scholar] [CrossRef]
- Hien, N.T.; Nguyen, L.H.; Van, H.T.; Nguyen, T.D.; Nguyen, T.H.V.; Chu, T.H.H.; Nguyen, T.V.; Trinh, V.T.; Vu, X.H.; Aziz, K.H.H. Heterogeneous Catalyst Ozonation of Direct Black 22 from Aqueous Solution in the Presence of Metal Slags Originating from Industrial Solid Wastes. Sep. Purif. Technol. 2020, 233, 115961. [Google Scholar] [CrossRef]
- Pereira, C.A.A.; Nava, M.R.; Walter, J.B.; Scherer, C.E.; Dominique Kupfer Dalfovo, A.; Barreto-Rodrigues, M. Application of Zero Valent Iron (ZVI) Immobilized in Ca-Alginate Beads for C.I. Reactive Red 195 Catalytic Degradation in an Air Lift Reactor Operated with Ozone. J. Hazard. Mater. 2021, 401, 123275. [Google Scholar] [CrossRef]
- Pervez, M.N.; Stylios, G.K.; Liang, Y.; Ouyang, F.; Cai, Y. Low-Temperature Synthesis of Novel Polyvinylalcohol (PVA) Nanofibrous Membranes for Catalytic Dye Degradation. J. Clean. Prod. 2020, 262, 121301. [Google Scholar] [CrossRef]
- Chen, C.; Jia, N.; Song, K.; Zheng, X.; Lan, Y.; Li, Y. Sulfur-Doped Copper-Yttrium Bimetallic Oxides: A Novel and Efficient Ozonation Catalyst for the Degradation of Aniline. Sep. Purif. Technol. 2020, 236, 116248. [Google Scholar] [CrossRef]
- Faghihinezhad, M.; Baghdadi, M.; Shahin, M.S.; Torabian, A. Catalytic Ozonation of Real Textile Wastewater by Magnetic Oxidized G-C3N4 Modified with Al2O3 Nanoparticles as a Novel Catalyst. Sep. Purif. Technol. 2022, 283, 120208. [Google Scholar] [CrossRef]
- Sone, B.T.; Makamu, E.; Mohamed, H.E.A.; Oputu, O.; Fester, V. Green-Synthesized ZnO via Hyphaene Thebaica Fruit Extracts: Structure & Catalytic Effect on the Ozonation of Coralene Rubine-S2G Azo Disperse Dye. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100515. [Google Scholar] [CrossRef]
- Yu, D.; Wu, M.; Hu, Q.; Wang, L.; Lv, C.; Zhang, L. Iron-Based Metal-Organic Frameworks as Novel Platforms for Catalytic Ozonation of Organic Pollutant: Efficiency and Mechanism. J. Hazard. Mater. 2019, 367, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Tariq, A.; Ikhlaq, A.; Rizvi, O.S.; Ikhlaq, U.; Masood, Z.; Qazi, U.Y.; Qi, F. Application of Laboratory-Grade Recycled Borosilicate Glass Coated with Iron and Cobalt for the Removal of Methylene Blue by Catalytic Ozonation Process. Arab. J. Sci. Eng. 2022, 1–16. [Google Scholar] [CrossRef]




| Fiber Type | Type of Dye | Auxiliaries | Temperature (Celsius) of Wastewater | pH of Wastewater |
|---|---|---|---|---|
| Cellulosic (cotton, viscose) | Reactive | dye | 60, 80 | 10–12 |
| Direct | salt (NaCl, Na2SO4) | |||
| soda (NaOH, Na2CO3) | ||||
| leveling agent | ||||
| the enzyme (against H2O2) | ||||
| Woll, Silk, Polyamide | Acid | Dye | 100 | 2–7 |
| acid (H2SO4, formic, acetic) | ||||
| salt (Na2SO4) | ||||
| ammonium sulfate | ||||
| Polyester | Disperse | Dye | 130 (under pressure) | 5 |
| Dispergator | ||||
| acid (formic, acetic) | ||||
| Acrylic | Cationic | Dye | 90 | 4–5 |
| acid (formic, acetic) | ||||
| salt (Na2SO4, sodium acetate) |
| Wastewater Type/Pollutant | Catalyst Type | Conditions | Removal | k (min−1) | Year, Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | C0 (mg/L) | O3 Dose (g/L) | Catal. Dose (g/L) | Color (%) | COD (%) | TOC (%) | ||||
| Dye solution Reactive Red 2 | Fe(II), Fe(III), Mn(II), Zn(II), Co(II), Ni(II) | 2 | 100 | - | 0.0335 | - | - | 13–23 (after 5 min) | 1.299 | 2008, [55] |
| 1.278 | ||||||||||
| 3.296 | ||||||||||
| 1.015 | ||||||||||
| 0.843 | ||||||||||
| 0.822 | ||||||||||
| Textile Effluent | Fe(II), nZVI | - | - | 0.05–0.2 | 0.7 | 87 (after 40 min) | 73.5 (after 40 min) | - | 0.000751 | 2018, [56] |
| 0.000948 | ||||||||||
| Reactive Red 120 | Fe(III) | 3 | 100 | - | 0.00558 | - | 40 (after 30 min) | - | - | 2012, [57] |
| Reactive Red 2 | Mn(II) | 2 | 100 | - | 0.1 | 95 (after 5 min) | - | 17–21 (after 5 min) | - | 2008, [58] |
| Wastewater Type/Pollutant | Catalyst Type | Conditions | Removal | k (min−1) | Year, Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | C0 (mg/L) | O3 Dose (g/L) | Catal. Dose (g/L) | Color (%) | COD (%) | TOC (%) | ||||
| Textile Wastewaters (Basic Blue 41, Basic Yellow 28, Basic Red 18.1) | Al2O3 | 4 | - | 0.0918 | 5 | - | Blue Dye—45.3; Red/Yellow Dyes—100 | - | - | 2015, [61] |
| Acid Red 151; | Al2O3 | 2.5 | 200 | 0.0147 | 5 | 98.4 (after 30 min) | 78.7 (after 30 min) | - | 0.136 | 2008, [62] |
| Remazol Blue R | 98.3 (after 30 min) | 82.6 (after 30 min) | - | 0.132 | ||||||
| Lemon Yellow | CeO2 | 6 | - | - | 0.5 | - | - | 97 | - | 2014, [63] |
| Reactive Black 5 | MgO | 7 | - | - | 0.05–2 | 99.9 | - | 38.8 | - | 2016, [64] |
| Reactive Red 198 | MgO | 8 | - | - | 1–6 | 100 | 69 | - | - | 2009, [65] |
| Methylene Blue | MgO | 9 | 500 | - | - | 77 (after 60 min) | 12.15 (after 60 min) | - | 0.025 | 2016, [66] |
| Reactive Red 2 | MnO2 | 2 | 100 | - | 0.8 | 95 (after 5 min) | - | 17–21 (after 5 min) | - | 2008, [58] |
| CI Acid Blue 113, aniline | Mn-O, Co-O, Ce-O | 3, 5.5, 6 | - | 0.05 | 0.35 | - | - | ~80 | - | 2009, [67] |
| Naphtol Blue Black | Co3O4, | 5 | - | - | 0.1 | 10–30 | - | 2007, [68] | ||
| Reactive Black 5 | Co3O4 | 7 | - | - | 0.2 | 99.99 | 80 | - | - | 2017, [69] |
| Industrial Wastewater | Fe-shaving based | 6.81 ± 0.14 | - | 0.065 | 3 | - | - | 98.5 | - | 2018, [70] |
| 2019, [71] | ||||||||||
| Acid Red 18 | Ca(OH)2 | 8 | - | 0.065 | 3 | ~100 | ~100 | - | 2017, [72] | |
| Wastewater Type/Pollutant | Catalyst Type | Conditions | Removal | k (min−1) | Year, Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | C0 (mg/L) | O3 Dose (g/L) | Catal. Dose (g/L) | Color (%) | COD (%) | TOC (%) | ||||
| Reactive Black 5 | Activated carbon | 11.29 | - | 0.9 | 0.005 | - | 40 (after 60 min) | 35 (after 60 min) | - | 2020, [75] |
| Aniline | Activated Carbon | 3 | 13.2 | 50 | 0.35 | - | - | - | 0.188, 0.290, 0.233 | 2007, [79] |
| 7 | ||||||||||
| 9 | ||||||||||
| Real textile effluent | Activated carbon | 8.5 | 100 | 0.00754 | 0.3 | ~100 (15 min) | - | 20.7 (after 60 min) | 0.47 | 2005, [87] |
| Reactive Blue 194 | Granular | 7 | 200 | 0.179 | - | 100 (after 20 min) | - | - | 2020, [51] | |
| activated carbon | ||||||||||
| Basic Blue 9 | Granular activated carbon | 10 | - | - | 2 | 80 (after 5 min) | 68 (after 5 min) | - | 0.72 | 2013, [77] |
| Reactive Red 195 | Granular activated carbon | 11 | 100 | - | 1 | 90.4 (after 2 min) | - | - | 2012, [80] | |
| Acid Red 3R | Granular Activated Carbon | 7 | 100 | 0.0417 | 2 | - | - | 78.2 (after 60 min) | - | 2018, [74] |
| C.I. Reactive Red 194, C.I. Reactive Yellow 145 | Granular Activated Carbon | 6.3 | 100 | 0.035 | 10 | ~100 (after 30 min) | 80 (after 30 min) | 50 (after 30 min) | - | 2009, [78] |
| 5.9 | ||||||||||
| Methylene Blue | Granular Activated Carbon | 9 | 500 | - | - | 63 (after 60 min) | 25.36 (after 60 min) | - | 0.016 | 2016, [66] |
| Bio-treated dyeing finishing wastewater | Regenerated granular activated carbon | - | - | 0.0185 | 2 | 81.7 (after 25 min) | 71 (after 25 min) | - | - | 2019, [76] |
| Reactive Red 198, | Multiwalled carbon nanotube | 3 | 150 | - | 0.03 | 100 (after 16 min), | - | - | - | 2013, [81] |
| Direct Green 6 | 100 (after 20 min) | |||||||||
| Indigo | Carbon nanotubes functionalized with carboxyl groups | 4 | 100 | 0.141 | 0.005 | ~99 (after 20 min) | - | 35.1 (after 2 h) | −0.219 | 2015, [82] |
| CI Acid Blue 113, | Activated carbon–cerium oxide | 5.6 | 50 | 50 | 0.35 | 97 | - | 88 (after 120 min) | - | 2009, [83] |
| CI Reactive Yellow 3 | 88 | |||||||||
| CI Reactive Blue 5 | 98 | |||||||||
| (after 5 min) | ||||||||||
| Dyeing effluents | Carbon aerogel-Co3O4 | 7, 10 | - | 0.008 | 3 | 100 | 80 (after 30 min) | - | - | 2016, [84] |
| (after 10 min) | ||||||||||
| Rhodamine B | Graphene/α-MnO2 nanocrystals hybrid aerogel (GMA) | - | 50 | 0.035 | - | 100 (after 60 min) | 89.02 | - | 0.2859 | 2019, [85] |
| (after 15 min) | ||||||||||
| Methyl Orange | Copper(II)-doped carbon dots | 7 | - | 1.98 | - | 99.8 (after 6 min) | - | - | 1.184 | 2022, [86] |
| Real textile effluent | 41 (after 60 min) | |||||||||
| 0.012 | ||||||||||
| Wastewater Type/Pollutant | Catalyst Type | Conditions | Removal | k (min−1) | Year, Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | C0 (mg/L) | O3 Dose (g/L) | Catal. Dose (g/L) | Color (%) | COD (%) | TOC (%) | ||||
| Methylene Blue, Methyl Green, Methyl Orange, Methylthymol Blue | Ion-exchanged montmorillonite, (NaMt and Fe(II)Mt), crude bentonite, and acid-activated counterparts (HMt) | - | 200 | 0.0083 | 0.04 | - | - | - | - | 2019, [89] |
| Methylene Blue | Volcanic sand | 8 | 30 | 0.006 | 50 | 70 | - | - | 0.09 | 2010, [91] |
| (after 50 min) | ||||||||||
| Methylene Blue | Zeolite | 2 | - | 15 | - | - | - | 0.054 | 2009, [93] | |
| Volcanic sand | 0.12 | |||||||||
| Basic Blue 3 | Natural Magnetite modified with argon plasma | 6.7 | 90 | 0.003 | 0.6 | 93.47 (after 15 min) | - | - | 0.1814 | 2015, [94] |
| Reactive Red-120 | Raw and Calcined magnetite | 11 | 100 | - | 3 | - | - | 96.1 (after 120 min) | 0.082 | 2012, [88] |
| Active Brilliant Red X-3B | Brucite | - | 500 | - | 0.5 | 89 | 32.5 | - | - | 2007, [92] |
| (after 15 min) | (after 15 min) | |||||||||
| Orange II | Aluminosilicate, Montanit300 | 6 | 100 | - | 1 | 100 (after 240 min) | - | 91 (after 240 min) | - | 2021, [90] |
| Wastewater Type/Pollutant | Catalyst Type | Conditions | Removal | k (min−1) | Year, Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | C0 (mg/L) | O3 Dose (g/L) | Catal. Dose (g/L) | Color (%) | COD (%) | TOC (%) | ||||
| Reactive Black 5 | La-Co-O | 3 | 100 | - | 0.005 | 99 (after 30 min) | - | - | - | 2020, [95] |
| Reactive Black 5 | Ag-La-Co | 7 | 100 | - | 0.5 | 95 (after 10 min) | - | 60 (after 80 min) | 0.0727 | 2021, [96] |
| Acid Red 151; | 100% perfluorooctyl alumina | 13 | 200 | 0.0147 | 5 | 98.8 (after 30 min), | 75.7 (after 30 min), | - | 0.132, | 2008, [62] |
| Remazol Blue R | 97.4 (after 30 min) | 96.6 (after 30 min) | - | 0.158 | ||||||
| Textile wastewater | C-doped MgO eggshell | - | - | 0.08 | 0.23 | 93 (after 10 min) | - | 78 (after 10 min) | 1.545 | 2019, [97] |
| C-MgO-EMP | ||||||||||
| Reactive Orange 4 | Cu/SBA-15 | 9 | 100 | 0.005 | 2 | 100 (after 21 min) | - | 86 (after 60 min) | 0.031 | 2018, [98] |
| Reactive Orange 4 | Mesoporous | 9 | 100 | 0.005 | 2 | 100 (after 21 min) | 70.4 (after 60 min), | - | - | 2018, [99] |
| Dye Industrial Effluent | Ru-Cu/SBA-15 | 90 (after 4 h) | ||||||||
| 7.5 | ||||||||||
| Dye wastewater | Fe/Mn@γ− Al2O3 | 7 | - | 0.006 | 0.2 | - | - | - | 0.132 | 2022, [102] |
| Basic Yellow 87 | Porous copper fiber sintered sheet | 6.6 | 216 | 0.5 | - | 100 (after 120 min) | 60 (after 120 min) | - | 2014, [100] | |
| Azo Dye 4BS | Mg-Ce membrane, | 8.5 | 12 | 0.012 | - | 85 (after 5 min) | - | >75 (after 5 min) | - | 2021, [101] |
| Mg-Mn membrane | 88 (after 5 min) | >75 (after 5 min) | ||||||||
| - | - | |||||||||
| Methylene Blue | G-MgO-SCCa-Zn | 9 | 500 | - | - | 98 (after 60 min) | 50 (after 60 min) | - | 0.04 | 2016, [66] |
| Acid Orange II | MgFe2O4 | 5 | 50 | 0.005 | 0.1 | - | - | 48.1 (after 40 min) | - | 2016, [103] |
| Methyl Orange (MO) | Ni-LDHs | 9 | 500 | - | 1 | 96 (after 60 min) | 72 (after 60 min) | - | 0.053 | 2019, [104] |
| Methyl Orange | NiFe2O4-NiO/NF | 6.84 | - | 0.0041 | - | 96 (after 60 min) | - | 71 (after 60 min) | - | 2021, [105] |
| Direct Black 22 | Zn-S | 3–11 | 100 | 3.38 | 0.75 | 99 (after 20 min) | 82 (after 25 min) | - | - | 2020, [106] |
| Reactive Red 195 | nZVI-Alg | 3.0–6.5 | 25 | 0.008 | 50 | 100 (after 30 min) | 98 (after 90 min) | - | - | 2021, [107] |
| Coralene Rubine-S2G | ZnO-400 nanoparticles | 6.8–8.4 | 130 | - | 0.050 | 100 (after 35 min) | - | - | 0.359 | 2021, [111] |
| Methylene Blue | Polyvinylalcohol (PVA) nanofibrous membranes | 3.02 | 20 | - | 0.03 | 94 (after 60 min) | - | - | - | 2020, [108] |
| Aniline | S-CuYO | 3–11 | 10 | - | 0–2.0 | 96 (after 15 min) | - | 57.7 (after 15 min) | - | 2020, [109] |
| Rhodamine B | Fe-MOFs | 7 | 40 | - | 0.2 | 100 (after 2.5 min) | - | 40 (after 30 min) | 5.76 | 2019, [112] |
| Real textile wastewater | O@g-C3N4/ Al2O3 | 7.1 | - | - | 0.5 | 99 (after 60 min) | 77 (after 60 min) | - | 0.155 | 2022, [110] |
| Methylene Blue | Co-BSG | 6.8 | 30 | - | 5 | 92 (after 8 min) | 93 (after 40 min) | - | - | 2022, [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilińska, M.; Bilińska, L.; Gmurek, M. Homogeneous and Heterogeneous Catalytic Ozonation of Textile Wastewater: Application and Mechanism. Catalysts 2023, 13, 6. https://doi.org/10.3390/catal13010006
Bilińska M, Bilińska L, Gmurek M. Homogeneous and Heterogeneous Catalytic Ozonation of Textile Wastewater: Application and Mechanism. Catalysts. 2023; 13(1):6. https://doi.org/10.3390/catal13010006
Chicago/Turabian StyleBilińska, Magdalena, Lucyna Bilińska, and Marta Gmurek. 2023. "Homogeneous and Heterogeneous Catalytic Ozonation of Textile Wastewater: Application and Mechanism" Catalysts 13, no. 1: 6. https://doi.org/10.3390/catal13010006
APA StyleBilińska, M., Bilińska, L., & Gmurek, M. (2023). Homogeneous and Heterogeneous Catalytic Ozonation of Textile Wastewater: Application and Mechanism. Catalysts, 13(1), 6. https://doi.org/10.3390/catal13010006