Solvent-Free Oxidation of Benzyl Alcohol Derivatives by In Situ Generated Redox Couple Pd(0)/PdOx Supported on Ceria Nanorods
Abstract
:1. Introduction
2. Results and Discussion
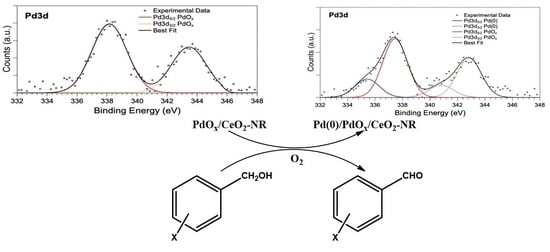
2.1. Characterization
2.2. Catalytic Tests
2.3. Mechanistic Aspects
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giang, L.T.K.; Ku, Y. Selective Oxidation of Benzyl Alcohol in Aqueous Phase by TiO2 Based Photocatalysts: A Review. Chem. Eng. Technol. 2021, 14, 2178–2190. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective Benzyl Alcohol Oxidation over Pd Catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Xi, J. Single-Atom Catalysis for Organic Reactions. Chin. Chem. Lett. 2022, 2022, 107959. [Google Scholar] [CrossRef]
- Xin, P.; Li, J.; Xiong, Y.; Wu, X.; Dong, J.; Chen, W.; Wang, Y.; Gu, L.; Luo, J.; Rong, H.; et al. Revealing the Active Species for Aerobic Alcohol Oxidation by Using Uniform Supported Palladium Catalysts. Angew. Chem. 2018, 130, 4732–4736. [Google Scholar] [CrossRef]
- Moeini, S.S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized Palladium Supported on Ceria Nanorods for Catalytic Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde in Protic Solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Moeini, S.S.; Pasqual Laverdura, U.; Marconi, E.; Lisi, N.; Serra, E.; Chierchia, R.; Luisetto, I.; Tuti, S.; Tofani, D. A Novel Pd-P Nano-Alloy Supported on Functionalized Silica for Catalytic Aerobic Oxidation of Benzyl Alcohol. Catalysts 2022, 12, 20. [Google Scholar] [CrossRef]
- Bourbiaux, D.; Mangematin, S.; Djakovitch, L.; Rataboul, F. Selective Aerobic Oxidation of Benzyl Alcohols with Palladium(0) Nanoparticles Suspension in Water. Catal. Lett. 2021, 151, 3239–3249. [Google Scholar] [CrossRef]
- Wang, H.; Kong, W.; Zhu, W.; Wang, L.; Yang, S.; Liu, F. One-Step Synthesis of Pd Nanoparticles Functionalized Crystalline Nanoporous CeO2 and Their Application for Solvent-Free and Aerobic Oxidation of Alcohols. Catal. Commun. 2014, 50, 87–91. [Google Scholar] [CrossRef]
- Xia, X.; Liu, S.; Long, Z.; Zhu, W.; Chen, G.; Huang, H.; Tong, M. P,N Co-Doped Biomass Carbon as a Remarkable Metal-Free Catalyst for Solvent-Free Oxidation of Benzyl Alcohol with Ambient Air: The Key Promoting Role of N Co-Doping. Appl. Surf. Sci. 2022, 571, 151409. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Yu, S.; Wei, Z.; Zhang, H. Highly Dispersed Pd Nanoclusters on Layered Double Hydroxides with Proper Calcination Improving Solvent-Free Oxidation of Benzyl Alcohol. ACS Sustain. Chem. Eng. 2022, 10, 7223–7233. [Google Scholar] [CrossRef]
- Yi, X.-T.; Li, C.-Y.; Wang, F.; Xu, J.; Xue, B. The Solvent-Free and Aerobic Oxidation of Benzyl Alcohol Catalyzed by Pd Supported on Carbon Nitride/CeO2 Composites. New J. Chem. 2022, 46, 7108–7117. [Google Scholar] [CrossRef]
- Nair, V.R.; Maiyalagan, T.; Shendage, S.S. Halloysite Clay Nanotubes with Fe–Al Deposits for the Oxidation of Benzyl Alcohol. New J. Chem. 2022, 46, 17213–17222. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Rossetti, I.; Villa, A.; Prati, L. Benzyl Alcohol Oxidation on Carbon-Supported Pd Nanoparticles: Elucidating the Reaction Mechanism. ChemCatChem 2014, 6, 3464–3473. [Google Scholar] [CrossRef]
- Galvanin, F.; Sankar, M.; Cattaneo, S.; Bethell, D.; Dua, V.; Hutchings, G.J.; Gavriilidis, A. On the Development of Kinetic Models for Solvent-Free Benzyl Alcohol Oxidation over a Gold-Palladium Catalyst. Chem. Eng. J. 2018, 342, 196–210. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, X.-T.; He, X.-Q.; Ji, H.-B. Mechanism and Kinetics of the Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde Catalyzed by Cobalt Porphyrin in a Membrane Microchannel Reactor. Chem. Eng. Sci. 2021, 245, 116847. [Google Scholar] [CrossRef]
- Takeyama, T.; Kobayashi, M.; Kikuchi, M.; Ogura, T.; Shimazaki, Y.; Iwatsuki, S. Benzyl Alcohol Oxidation Mechanisms by One- and Two-Electron Oxidized Species of Cu(II)-Salen Complexes with Para-R-Substituents, [Cu(R-Salen)]N+ (R = MeO, MeS; n = 1, 2). Inorg. Chim. Acta 2020, 511, 119848. [Google Scholar] [CrossRef]
- Arena, F.; Gumina, B.; Cannilla, C.; Spadaro, L.; Patti, A.; Spiccia, L. Nanostructured MnOx Catalysts in the Liquid Phase Selective Oxidation of Benzyl Alcohol with Oxygen: Part II. Reaction Mechanism, Kinetics and Deactivation Pattern. Appl. Catal. B Environ. 2015, 170–171, 233–240. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Qayyum, A.; Barczak, M.; Colmenares-Quintero, R.F.; Borowski, P.; Triantafyllidis, K.; Colmenares, J.C. Mechanistic and Kinetic Studies of Benzyl Alcohol Photocatalytic Oxidation by Nanostructured Titanium (Hydro) Oxides: Do We Know the Entire Story? Appl. Catal. B Environ. 2023, 320, 121939. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.-K.; Chen, Y.; Wang, Y.; Li, Y.-X. Palladium Nanoparticles Supported on Mesoporous Carbon Nitride for Efficiently Selective Oxidation of Benzyl Alcohol with Molecular Oxygen. Appl. Catal. Gen. 2017, 542, 380–388. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Caravati, M.; Baiker, A. Oxidic or Metallic Palladium: Which Is the Active Phase in Pd-Catalyzed Aerobic Alcohol Oxidation? J. Phys. Chem. B 2006, 110, 25586–25589. [Google Scholar] [CrossRef]
- Wu, P.; Cao, Y.; Zhao, L.; Wang, Y.; He, Z.; Xing, W.; Bai, P.; Mintova, S.; Yan, Z. Formation of PdO on Au–Pd Bimetallic Catalysts and the Effect on Benzyl Alcohol Oxidation. J. Catal. 2019, 375, 32–43. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Jürgensen, A.; Heutz, N.; Raschke, H.; Merz, K.; Hergenröder, R. Behavior of Supported Palladium Oxide Nanoparticles under Reaction Conditions, Studied with near Ambient Pressure XPS. Anal. Chem. 2015, 87, 7848–7856. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Shyu, J.Z.; Weber, W.H.; Gandhi, H.S. Surface Characterization of Alumina-Supported Ceria. J. Phys. Chem. 1988, 92, 4964–4970. [Google Scholar] [CrossRef]
- Szijjártó, G.P.; Pászti, Z.; Sajó, I.; Erdőhelyi, A.; Radnóczi, G.; Tompos, A. Nature of the Active Sites in Ni/MgAl2O4-Based Catalysts Designed for Steam Reforming of Ethanol. J. Catal. 2013, 305, 290–306. [Google Scholar] [CrossRef]
- Danielis, M.; Betancourt, L.E.; Orozco, I.; Divins, N.J.; Llorca, J.; Rodríguez, J.A.; Senanayake, S.D.; Colussi, S.; Trovarelli, A. Methane Oxidation Activity and Nanoscale Characterization of Pd/CeO2 Catalysts Prepared by Dry Milling Pd Acetate and Ceria. Appl. Catal. B Environ. 2021, 282, 119567. [Google Scholar] [CrossRef]
- Gopinath, R.; Lingaiah, N.; Sreedhar, B.; Suryanarayana, I.; Sai Prasad, P.S.; Obuchi, A. Highly Stable Pd/CeO2 Catalyst for Hydrodechlorination of Chlorobenzene. Appl. Catal. B Environ. 2003, 46, 587–594. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, G.; Lee, H. Continuous Methane to Ethane Conversion Using Gaseous Oxygen on Ceria-Based Pd Catalysts at Low Temperatures. Appl. Catal. Gen. 2021, 623, 118245. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.; Ma, K.; Zhang, Y.; Hu, Y.-M.; Kong, L.; Jia, A.; Zhang, Z.; Huang, W.; Lu, J.-Q. Ceria Morphology-Dependent Pd-CeO2 Interaction and Catalysis in CO2 Hydrogenation into Formate. J. Catal. 2021, 397, 116–127. [Google Scholar] [CrossRef]
- Yan, Z.; Tomer, A.; Perrussel, G.; Ousmane, M.; Katryniok, B.; Dumeignil, F.; Ponchel, A.; Liebens, A.; Pera-Titus, M. A Pd/CeO2 “H2 Pump” for the Direct Amination of Alcohols. ChemCatChem 2016, 8, 3347–3352. [Google Scholar] [CrossRef]
- Luo, M.-F.; Hou, Z.-Y.; Yuan, X.-X.; Zheng, X.-M. Characterization Study of CeO2 Supported Pd Catalyst for Low-Temperature Carbon Monoxide Oxidation. Catal. Lett. 1998, 50, 205–209. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Meng, M.; Xian, H.; Tu, Y.-B.; Li, X.-G.; Ding, T. The Nanomorphology-Controlled Palladium-Support Interaction and the Catalytic Performance of Pd/CeO2 Catalysts. Catal. Lett. 2009, 133, 328–333. [Google Scholar] [CrossRef]
- Neal, L.M.; Everett, M.L.; Hoflund, G.B.; Hagelin-Weaver, H.E. Characterization of Palladium Oxide Catalysts Supported on Nanoparticle Metal Oxides for the Oxidative Coupling of 4-Methylpyridine. J. Mol. Catal. Chem. 2011, 335, 210–221. [Google Scholar] [CrossRef]
- Kara, G.K.; Rahimi, J.; Niksefat, M.; Taheri-Ledari, R.; Rabbani, M.; Maleki, A. Preparation and Characterization of Perlite/V2O5 Nano-Spheres via a Novel Green Method: Applied for Oxidation of Benzyl Alcohol Derivatives. Mater. Chem. Phys. 2020, 250, 122991. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic Oxidation of Lignin in Solvent Systems for Production of Renewable Chemicals: A Review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective Photocatalytic Oxidation of Benzyl Alcohol and Its Derivatives into Corresponding Aldehydes by Molecular Oxygen on Titanium Dioxide under Visible Light Irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Goksu, H.; Sen, F. Handy and Highly Efficient Oxidation of Benzylic Alcohols to the Benzaldehyde Derivatives Using Heterogeneous Pd/AlO(OH) Nanoparticles in Solvent-Free Conditions. Sci. Rep. 2020, 10, 5731. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, B.; Yang, S.; Yang, X.; Meng, F.; Zhai, L.; Li, Z.; Zhao, S.; Zhang, G.; Qin, Y. Dual Pd2+ and Pd0 Sites on CeO2 for Benzyl Alcohol Selective Oxidation. J. Catal. 2022, 414, 385–393. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1. Available online: https://srdata.nist.gov/xps/ (accessed on 13 November 2022).
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple Preparation of Nitrogen-Doped TiO2 and Its Performance in Selective Oxidation of Benzyl Alcohol and Benzylamine under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125743. [Google Scholar] [CrossRef]
- Tuti, S.; Luisetto, I.; Pasqual Laverdura, U.; Marconi, E. Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability. Reactions 2022, 3, 333–351. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; ISBN 978-0-9627026-2-4. [Google Scholar]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Hantsche, H. High Resolution XPS of Organic Polymers, the Scienta ESCA300 Database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., Hardcover, ISBN 0-471-93592-1. Adv. Mater. 1993, 5, 778. [Google Scholar] [CrossRef]






| Sample | CeO2 Crystallite Size (nm) | CeO2 Cell Size (Å) | Surface Area (m2 g−1) | Average Pore Diameter (nm) | Total Pore Volume (cm3 g−1) |
|---|---|---|---|---|---|
| CeO2-NR | 13 | 5.408 ± 0.001 | 94 | 19 | 0.40 |
| PdOx/CeO2-NR | 12 | 5.405 ± 0.003 | 68 | 13 | 0.26 |
| Signal | BE (eV) | FWHM (eV) | Assignment |
|---|---|---|---|
| Ce3d5/2 | 882.59 | 3.87 | Ce(III) |
| - | 885.20 | 3.87 | Ce(IV) |
| - | 887.24 | 3.87 | Ce(III) |
| - | 891.14 | 3.87 | Ce(IV) |
| - | 898.72 | 3.87 | Ce(IV) |
| Pd3d5/2 | 338.16 | 2.76 | PdOx |
| Sample | H2 Consumption in Range 40–300 °C (μmol g−1) | e−/Pd (mol/mol) | H2 Consumption (T Range) (μmol g−1) | Ce(III) (%) |
|---|---|---|---|---|
| CeO2-NR | - | - | 518 (250–500 °C) | 17.8 |
| PdOx/CeO2-NR | 175 | 1.87 | 19 (300–500 °C) | 0.64 |
| Entry | Reactant | Conversion (%) | Aldehyde Selectivity (%) |
|---|---|---|---|
| 1 |  | 50 | 93 |
| 2 |  | 70 | 86 |
| 3 |  | 46 | 97 |
| 4 |  | 52 | 90 |
| 5 |  | <3 | >97 |
| 6 |  | 66 | 81 |
| 7 |  | <3 | >97 |
| 8 |  | 62 | 94 |
| 9 |  | >97 | <3 |
| 10 |  | <3 | >97 |
| 11 |  | <3 | >97 |
| 12 |  | 31 | <3 |
| Reaction Cycle | BnOH Conversion % | PhCHO Selevtivity % |
|---|---|---|
| 1 | 51 | 94 |
| 2 | 61 | 89 |
| 3 | 60 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moeini, S.S.; Tuti, S.; Battocchio, C.; Luisetto, I.; Tofani, D. Solvent-Free Oxidation of Benzyl Alcohol Derivatives by In Situ Generated Redox Couple Pd(0)/PdOx Supported on Ceria Nanorods. Catalysts 2023, 13, 5. https://doi.org/10.3390/catal13010005
Moeini SS, Tuti S, Battocchio C, Luisetto I, Tofani D. Solvent-Free Oxidation of Benzyl Alcohol Derivatives by In Situ Generated Redox Couple Pd(0)/PdOx Supported on Ceria Nanorods. Catalysts. 2023; 13(1):5. https://doi.org/10.3390/catal13010005
Chicago/Turabian StyleMoeini, Seyed Sepehr, Simonetta Tuti, Chiara Battocchio, Igor Luisetto, and Daniela Tofani. 2023. "Solvent-Free Oxidation of Benzyl Alcohol Derivatives by In Situ Generated Redox Couple Pd(0)/PdOx Supported on Ceria Nanorods" Catalysts 13, no. 1: 5. https://doi.org/10.3390/catal13010005
APA StyleMoeini, S. S., Tuti, S., Battocchio, C., Luisetto, I., & Tofani, D. (2023). Solvent-Free Oxidation of Benzyl Alcohol Derivatives by In Situ Generated Redox Couple Pd(0)/PdOx Supported on Ceria Nanorods. Catalysts, 13(1), 5. https://doi.org/10.3390/catal13010005