Investigation of CeO2, MoO3, and Ce2(MoO4)3, Synthesized by the Pechini Method, as Catalysts for Fructose Conversion
Abstract
:1. Introduction
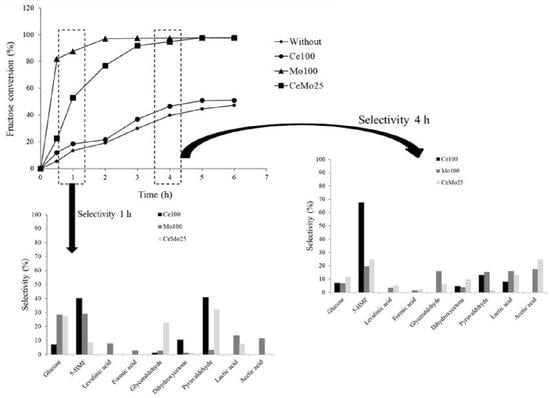
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization
3.3. Conversion of Fructose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Gao, Y.; Chen, H.; Wu, Y.; Wang, J.; Yu, C.; Li, J.; Zou, C. Biomass Briquette Fuel, Boiler Types and Pollutant Emissions of Industrial Biomass Boiler: A Review. Particuology 2022, 77, 79–90. [Google Scholar] [CrossRef]
- Gani, A. Fossil Fuel Energy and Environmental Performance in an Extended STIRPAT Model. J. Clean. Prod. 2021, 297, 126526. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2022; in press. [Google Scholar] [CrossRef]
- Bayu, A.; Abudula, A.; Guan, G. Reaction Pathways and Selectivity in Chemo-Catalytic Conversion of Biomass-Derived Carbohydrates to High-Value Chemicals: A Review. Fuel Process. Technol. 2019, 196, 106162. [Google Scholar] [CrossRef]
- Mehta, J.; Metre, A.V.; Bhakhar, M.S.; Panwar, D.S.; Dharaskar, S. Biomass-Derived 5-Hydroxymethylfurfural (HMF) and 2,5-Dimethylfuran (DMF) Synthesis as Promising Alternative Fuel: A Prospective Review. Mater. Today Proc. 2022, 62, 6978–6984. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Jin, D.; Jiao, G.; Yang, X.; Liu, K.; Zhou, J.; Sun, R. Copper Oxide Functionalized Chitosan Hybrid Hydrogels for Highly Efficient Photocatalytic-Reforming of Biomass-Based Monosaccharides to Lactic Acid. Appl. Catal. 2021, 291, 120123. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Rakić, V.; Auroux, A. CeO2–Nb2O5 Mixed Oxide Catalysts: Preparation, Characterization and Catalytic Activity in Fructose Dehydration Reaction. Catal. Today 2012, 192, 160–168. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of Mixed Metal Oxides in Catalysis Science—Versatile Applications in Organic Synthesis. Catal. Sci. Technol. 2012, 2, 1113. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Li, W.; Shen, P.; Wu, Y.; Song, X.; Zhu, Y.; Xu, N.; Gao, L.; Xiao, G. Direct Conversion of Biomass-Derived Carbohydrates to 5-Hydroxymethylfurfural Using na Efficient and Inexpensive Manganese Phosphate Catalyst. Fuel Process. Technol. 2018, 181, 199–206. [Google Scholar] [CrossRef]
- Dos Santos, T.V.; Da Silva Avelino, D.O.; Meneghetti, M.R.; Meneghetti, S.M.P. Mixed Oxides Based on SnO2 Impregnated with MoO3: A Robust System to Apply in Fructose Conversion. Catal. Commun. 2018, 114, 120–123. [Google Scholar] [CrossRef]
- Oliveira, F.K.F.; Santiago, A.A.G.; Catto, A.C.; da Silva, L.F.; Tranquilin, R.L.; Longo, E.; Motta, F.V.; Bomio, M.R.D. Cerium Molybdate Nanocrystals: Microstructural, Optical and Gas-Sensing Properties. J. Alloy. Compd. 2021, 857, 157562. [Google Scholar] [CrossRef]
- Chen, B.; Li, P.; Wang, B.; Wang, Y. Flame-Annealed Porous TiO2/CeO2 Nanosheets for Enhenced CO Gas Sensors. Appl. Surf. Sci. 2022, 593, 153418. [Google Scholar] [CrossRef]
- Benali, F.; Boukoussa, B.; Ismail, I.; Hachemaoui, M.; Iqbal, J.; Taha, I.; Cherifi, Z.; Mokhtar, A. One Pot Preparation of CeO2@Alginate Composite Beads for the Catalytic Reduction of MB Dye: Effect of Cerium Percentage. Surf. Interfaces 2021, 26, 101306. [Google Scholar] [CrossRef]
- Assis, G.C.; Silva, I.M.A.; Dos Santos, T.V.; Meneghetti, M.R.; Meneghetti, S.M.P. Photocatalytic Properties of SnO2/MoO3 Mixed Oxides and Their Relation to the Electronic Properties and Surface Acidity. J. Photochem. Photobiol. 2021, 407, 113035. [Google Scholar] [CrossRef]
- Muthuvel, I.; Sathyapriya, S.; Suguna, S.; Gowthami, K.; Thirunarayanan, G.; Rajalakshmi, S.; Sundaramurthy, N.; Dinesh Karthik, A.; Rajachandrasekar, T. Solar Light Driven Cerium Molybdate Nanocatalyst for Effective Photodecomposition of Fuchsin Basic Dye. Mater. Today Proc. 2021, 43, 2274–2279. [Google Scholar] [CrossRef]
- Ito, T.; Sunada, K.; Nagai, T.; Ishiguro, H.; Nakano, R.; Suzuki, Y.; Nakano, A.; Yano, H.; Isobe, T.; Matsushita, S.; et al. Preparation of Cerium Molybdates and Their Antiviral Activity against Bacteriophage Φ6 and SARS-CoV-2. Mater. Lett. 2021, 290, 129510. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Li, X.-P.; Zhao, R.-X. Ce2(MoO4)3 as na Efficient Catalyst for Aerobic Oxidative Desulfurization of Fuels. Pet. Sci. 2022, 19, 861–869. [Google Scholar] [CrossRef]
- Putri, G.E.; Rilda, Y.; Syukri, S.; Labanni, A.; Arief, S. Highly Antimicrobial Activity of Cerium Oxide Nanoparticles Synthesized Using Moringa Oleifera Leaf Extract by a Rapid Green Precipitation Method. J. Mater. Res. Technol. 2021, 15, 2355–2364. [Google Scholar] [CrossRef]
- Hadebe, N.P.; Kerru, N.; Tukulula, M.; Jonnalagadda, S.B. A Sustainable Molybdenum Oxide Loaded on Zirconia (MoO3/ZrO2) Catalysed Multicomponent Reaction to Synthesise Novel Dihydropyridines. Sustain. Chem. Pharm. 2022, 25, 100578. [Google Scholar] [CrossRef]
- Afza, N.; Shivakumar, M.S.; Alam, M.W.; Kumar, A.N.; Bhatt, A.S.; Murthy, H.C.A.; Ravikumar, C.R.; Mylarappa, M.; Selvanandan, S. Facile Hydrothermal Synthesis of Cerium Oxide/RGO Nanocomposite for Photocatalytic and Supercapacitor Applications. Appl. Surf. Sci. 2022, 11, 100307. [Google Scholar] [CrossRef]
- Garcia, A.B.S.; Bispo-Jr, A.G.; Lima, S.A.M.; Pires, A.M. Effects of the Pechini’s Modified Synthetic Route on Structural and Photophysical Properties of Eu3+ or Tb3+-Doped LaAlO3. Mater. Res. Bull. 2021, 143, 111462. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Huang, D.; Wang, X.; Cai, L.; Chen, Y.; Wang, W.; Song, Y.; Han, G.; Zhen, B. A High-Performance Ethanol Gas Sensor Based on Ce-Doped SnO2 Nanomaterials Prepared by the Pechini Method. Mater. Sci. Semicond. Process. 2022, 137, 106188. [Google Scholar] [CrossRef]
- Dos Santos, T.V.; Pryston, D.B.A.; Assis, G.C.; Meneghetti, M.R.; Meneghetti, S.M.P. Tin, Niobium and Tin-Niobium Oxides Obtained by the Pechini Method Using Glycerol as a Polyol: Synthesis, Characterization and Use as a Catalyst in Fructose Conversion. Catal. Today 2021, 379, 62–69. [Google Scholar] [CrossRef]
- Kang, J.; Gwon, Y.R.; Cho, S.K. Photoelectrochemical Water Oxidation on PbCrO4 Thin Film Photoanode Fabricated via Pechini Method: Various Solution-Processes for PbCrO4 Film Synthesis. J. Electroanal. Chem. 2020, 878, 114601. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Jasim, L.S.; Ranjeh, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Improved Pechini Sol-Gel Fabrication of Li2B4O7/NiO/Ni3(BO3)2 Nanocomposites to Advanced Photocatalytic Performance. Arab. J. Chem. 2022, 15, 103768. [Google Scholar] [CrossRef]
- Aflaki, M.; Davar, F. Synthesis, luminescence and photocatalyst properties of zirconia nanosheets by modified Pechini method. J. Mol. Liq. 2016, 221, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Bayat, B.; Tahvildari, K.; Hemmati, A.; Bazyari, A.; Ghaemi, A. Production of ethylene glycol monobutyl ether through etherification of ethylene glycol using a nanostructured heterogeneous catalyst of amyberlyst-15. Process Saf. Environ. Prot. 2022, 165, 597–609. [Google Scholar] [CrossRef]
- Mendelson, N.L.; Fitzgerald, A.; Kumar, A.; Pineda, J.A.; MacDougall, J.; Solomon, R.J.; Gibson, P.C.; Prikis, M.; Marroquin, C.E. Impact of Ethylene Glycol Toxicity on Donor Organ Viability: A Case Report. Transplant. Proc. 2017, 49, 2411–2414. [Google Scholar] [CrossRef]
- Pirzadi, Z.; Meshkani, F. From glycerol production to its value-added uses: A critical review. Fuel 2022, 329, 125044. [Google Scholar] [CrossRef]
- Durán, P.; Capel, F.; Gutierrez, D.; Tartaj, J.; Moure, C. Cerium (IV) Oxide Synthesis and Sinterable Powders Prepared by the Polymeric Organic Complex Solution Method. J. Eur. Ceram. Soc. 2002, 22, 1711–1721. [Google Scholar] [CrossRef]
- Bodachivskyi, I.; Kuzhiumparambil, U.; Williams, D.B.G. Acid-Catalyzed Conversion of Carbohydrates intoValue-Added Small Molecules in Aqueous Media and Ionic Liquids. ChemSusChem 2018, 11, 642–660. [Google Scholar] [CrossRef] [PubMed]
- Doungsri, S.; Rattanaphanee, P.; Wongkoblap, A. Production of Lactic Acid from Cellulose Using Solid Catalyst. MATEC Web Conf. 2019, 268, 07006. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Catalytic Dehydration of Fructose into 5-Hydroxymethylfurfural by Ion-Exchange Resin in Mixed-Aqueous System by Microwave Heating. Green Chem. 2008, 10, 799. [Google Scholar] [CrossRef]
- Gomes, F.N.D.C.; Mendes, F.M.T.; Souza, M.M.V.M. Synthesis of 5-Hydroxymethylfurfural from Fructose Catalyzed by Phosphotungstic acid. Catal. Today 2017, 279, 296–304. [Google Scholar] [CrossRef]
- Taghavi, S.; Ghedini, E.; Menegazzo, F.; Mäki-Arvela, P.; Peurla, M.; Zendehdel, M.; Cruciani, G.; Di Michele, A.; Murzin, D.Y.; Signoretto, M. CuZSM-5@HMS Composite as an Efficient Micro-Mesoporous Catalyst for Conversion of Sugars into Levulinic Acid. Catal. Today 2022, 390, 146–161. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Bai, X.; Du, Y. Conversion of Biomass into 5-Hydroxymethylfurfural Using Solid Acid Catalyst. Bioresour. Technol. 2011, 102, 3424–3429. [Google Scholar] [CrossRef]
- Wei, W.; Yang, H.; Wu, S. Efficient Conversion of Carbohydrates into Levulinic Acid over Chromium Modified Niobium Phosphate Catalyst. Fuel 2019, 256, 115940. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Orella, M.J.; Lin, Z.; Cheng, Z.; Zheng, W.; Nikolakis, V.; Vlachos, D.G. Molecular Structure, Morphology and Growth Mechanisms and Rates of 5-Hydroxymethyl Furfural (HMF) Derived Humins. Green Chem. 2016, 18, 1983–1993. [Google Scholar] [CrossRef] [Green Version]
- Cantero, D.A.; Vaquerizo, L.; Martinez, C.; Bermejo, M.D.; Cocero, M.J. Selective Transformation of Fructose and High Fructose Content Biomass into Lactic Acid in Supercritical Water. Catal. Today 2015, 255, 80–86. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The Mechanism for Thermal Decomposition of Cellulose and Its Main Products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Liu, M.; Li, Z.; Xiong, L.; Zhang, D.; Meng, K.; Qu, X.; Zhang, G.; Jin, X.; Yang, C. Hydrothermal Conversion of Fructose to Lactic Acid and Derivatives: Synergies of Metal and Acid/Base Catalysts. Chin. J. Chem. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Kong, L.; Shen, Z.; Zhang, W.; Xia, M.; Gu, M.; Zhou, X.; Zhang, Y. Conversion of Sucrose into Lactic Acid over Functionalized Sn-Beta Zeolite Catalyst by 3-Aminopropyltrimethoxysilane. ACS Omega 2018, 3, 17430–17438. [Google Scholar] [CrossRef] [PubMed]
- Lomate, S.; Katryniok, B.; Dumeignil, F.; Paul, S. High Yield Lactic Acid Selective Oxidation into Acetic Acid over a Mo-V-Nb Mixed Oxide Catalyst. Sustain. Chem. Process. 2015, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Zeng, X.; Yan, P.; Jing, Z.; Jin, F. An Acidic Two-Step Hydrothermal Process To Enhance Acetic Acid Production from Carbohydrate Biomass. Ind. Eng. Chem. Res. 2012, 51, 4759–4763. [Google Scholar] [CrossRef]
- Dos Santos, T.V.; Da Silva Avelino, D.O.; Pryston, D.B.A.; Meneghetti, M.R.; Meneghetti, S.M.P. Tin, molybdenum and tin-molybdenum oxides: Influence of Lewis and Bronsted acid sites on xylose conversion. Catal. Today 2021, 394, 125–132. [Google Scholar] [CrossRef]
- Yousefi, T.; Khanchi, A.R.; Ahmadi, S.J.; Rofouei, M.K.; Yavari, R.; Davarkhah, R.; Myanji, B. Cerium(III) Molybdate Nanoparticles: Synthesis, Characterization and Radionuclides Adsorption Studies. J. Hazard. Mater. 2012, 215, 266–271. [Google Scholar] [CrossRef]
- Nasser, H.; Rédey, Á.; Yuzhakova, T.; Kovács, J. Thermal Stability and Surface Structure of Mo/CeO2 and Ce-Doped Mo/Al2O3 Catalysts. J. Therm. Anal. Calorim. 2009, 95, 69–74. [Google Scholar] [CrossRef]
- Castellan, A.; Bart, J.C.J.; Bossi, A.; Perissinoto, P.; Giordano, N. On the Formation of Cerium Molybdates under different atmospheric and thermal conditions. Z. Anorg. Allg. Chem. 1976, 422, 155–172. [Google Scholar] [CrossRef]
- Khalil, K.M.S.; Elkabee, L.A.; Murphy, B. Preparation and Characterization of Thermally Stable Porous Ceria Aggregates Formed via a Sol–Gel Process of Ultrasonically Dispersed Cerium(IV) Isopropoxide. Micropor. Mesopor. Mater. 2005, 78, 83–89. [Google Scholar] [CrossRef]
- Suresh, R.; Ponnuswamy, V.; Mariappan, R. Effect of Annealing Temperature on the Microstructural, Optical and Electrical Properties of CeO2 Nanoparticles by Chemical Precipitation Method. Appl. Surf. Sci. 2013, 273, 457–464. [Google Scholar] [CrossRef]



 = fluorite-like face-centered cubic structure (JCPDS 34-0394)), Mo100 (* = orthorhombic phase (JCPDS No. 05-0508)), and CeMo (
= fluorite-like face-centered cubic structure (JCPDS 34-0394)), Mo100 (* = orthorhombic phase (JCPDS No. 05-0508)), and CeMo ( = Ce2(MoO4)3 (JCPDS 30–0303).
= Ce2(MoO4)3 (JCPDS 30–0303).
 = fluorite-like face-centered cubic structure (JCPDS 34-0394)), Mo100 (* = orthorhombic phase (JCPDS No. 05-0508)), and CeMo (
= fluorite-like face-centered cubic structure (JCPDS 34-0394)), Mo100 (* = orthorhombic phase (JCPDS No. 05-0508)), and CeMo ( = Ce2(MoO4)3 (JCPDS 30–0303).
= Ce2(MoO4)3 (JCPDS 30–0303).

| Sample | SBET a (m2·g−1) | Crystallite Size (nm) b | Amount Bronsted c (mmol·g−1) | Amount Lewis c (mmol·g−1) | AB/AL d |
|---|---|---|---|---|---|
| Ce100 | 70.6 | 10.1 | 157.5 | 120.0 | 1.3 |
| Mo100 | >5.0 | 133.6 | 700.0 | 531.8 | 1.3 |
| CeMo | >5.0 | 40.5 | 490.7 | 291.1 | 1.7 |
| Range T (°C) | Ce100 | Mo100 | CeMo |
|---|---|---|---|
| 100–150 | 2.3 | nd | nd |
| 700–850 | 2.7 | 78.2 | nd |
| 850–1000 | nd | 17.5 | nd |
| >1000 | 2.4 | nd | 19.0 |
| Code | Description |
|---|---|
| Ce100 | Cerium oxide(IV) (CeO2) |
| Mo100 | Molybdenum oxide(VI) (MoO3) |
| CeMo | Cerium molybdate (Ce2(MoO4)3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryston, D.B.d.A.; Martins, T.V.d.S.; Vasconcelos Júnior, J.A.d.; Avelino, D.O.d.S.; Meneghetti, M.R.; Meneghetti, S.M.P. Investigation of CeO2, MoO3, and Ce2(MoO4)3, Synthesized by the Pechini Method, as Catalysts for Fructose Conversion. Catalysts 2023, 13, 4. https://doi.org/10.3390/catal13010004
Pryston DBdA, Martins TVdS, Vasconcelos Júnior JAd, Avelino DOdS, Meneghetti MR, Meneghetti SMP. Investigation of CeO2, MoO3, and Ce2(MoO4)3, Synthesized by the Pechini Method, as Catalysts for Fructose Conversion. Catalysts. 2023; 13(1):4. https://doi.org/10.3390/catal13010004
Chicago/Turabian StylePryston, Dhara Beatriz de Amorim, Thatiane Veríssimo dos Santos Martins, Jailton Alves de Vasconcelos Júnior, Débora Olimpio da Silva Avelino, Mario Roberto Meneghetti, and Simoni Margareti Plentz Meneghetti. 2023. "Investigation of CeO2, MoO3, and Ce2(MoO4)3, Synthesized by the Pechini Method, as Catalysts for Fructose Conversion" Catalysts 13, no. 1: 4. https://doi.org/10.3390/catal13010004
APA StylePryston, D. B. d. A., Martins, T. V. d. S., Vasconcelos Júnior, J. A. d., Avelino, D. O. d. S., Meneghetti, M. R., & Meneghetti, S. M. P. (2023). Investigation of CeO2, MoO3, and Ce2(MoO4)3, Synthesized by the Pechini Method, as Catalysts for Fructose Conversion. Catalysts, 13(1), 4. https://doi.org/10.3390/catal13010004