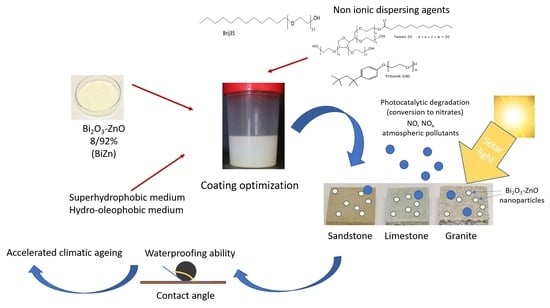
Development of Photocatalytic Coatings for Building Materials with Bi2O3-ZnO Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Photocatalytic Nanoparticles
2.2. Influence of the Non-Ionic Dispersing Agents on the Distribution of the Photocatalytic Nanoparticles
2.3. Characterization of the Coatings
2.4. Durability of the Coatings
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Characterization of Nanoparticles
3.2.2. Preparation of Multifunctional Active Coatings and Evaluation of Their Efficiency
- If the liquid fully penetrates the cavities, it is referred to as a Wenzel situation.
- If the liquid cannot expel the air trapped within the cavities, it is termed a Cassie–Baxter situation.
3.2.3. Durability of the Coatings after Accelerated Climatic Ageing
- Maintaining conditions at 35 °C, with exposure to UV-VIS radiation and a relative humidity of 40%;
- Reducing temperature to 20 °C with a relative humidity of 90%, simulating rainwater exposure;
- Lowering the temperature further to 0 °C with a relative humidity of 60%;
- Extending the simulation to −5 °C with a relative humidity of 50%.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brimblecombe, P.; Grossi, C.M. Aesthetic thresholds and blackening of stone buildings. Sci. Total Environ. 2005, 349, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, D.; Villa, F.; Cappitelli, F.; Toniolo, L. Biofilm colonization of metamorphic lithotypes of a renaissance cathedral exposed to urban atmosphere. Sci. Total Environ. 2018, 639, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Antonio, J.S.; Puente, I.; Pereira, M.F.C.; Rocha, C.S.A. Quantification and mapping of deterioration patterns on granite surfaces by means of mobile LiDAR data. Measurement 2019, 140, 227–236. [Google Scholar] [CrossRef]
- Vidal, F.; Vicente, R.; Mendes Silva, J. Review of environmental and air pollution impacts on built heritage: 10 questions on corrosion and soiling effects for urban intervention. J. Cult. Herit. 2019, 37, 273–295. [Google Scholar] [CrossRef]
- Brai, M.; Camaiti, M.; Casieri, C.; De Luca, F.; Fantazzini, P. Nuclear magnetic resonance for cultural heritage. Magn. Reson. Imaging 2007, 25, 461–465. [Google Scholar] [CrossRef]
- Piuzzi, E.; Pittella, E.; Pisa, S.; Cataldo, A.; De Benedetto, E.; Cannazza, G. An improved noninvasive resonance method for water content characterization of Cultural Heritage stone materials. Measurement 2018, 125, 257–261. [Google Scholar] [CrossRef]
- Gomes, V.; Dionísio, A.; Pozo-Antonio, J.S.; Rivas, T.; Ramil, A. Mechanical and laser cleaning of spray graffiti paints on a granite subjected to a SO2-rich atmosphere. Constr. Build. Mater. 2018, 188, 621–632. [Google Scholar] [CrossRef]
- Sanmartín, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Fermo, P.; Cappelletti, G.; Cozzi, N.; Padeletti, G.; Kaciulis, S.; Brucale, M.; Merlini, M. Hydrophobizing coatings for cultural heritage. A detailed study of resin/stone surface interaction. Appl. Phys. A 2014, 116, 341–348. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Alessandrini, G.; Aglietto, M.; Castelvetro, V.; Ciardelli, F.; Peruzzi, R.; Toniolo, L. Comparative evaluation of fluorinated and unfluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000, 76, 962–977. [Google Scholar] [CrossRef]
- Andreotti, S.; Franzoni, E.; Fabbri, P. Poly(hydroxyalkanoate)s-Based Hydrophobic Coatings for the Protection of Stone in Cultural Heritage. Materials 2018, 11, 165. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne Superhydrophobic and Superoleophobic Coatings for the Protection of Marble and Sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef]
- Winandy, L.; Schlebusch, O.; Fischer, R. Fungal hydrophobins render stones impermeable for water but keep them permeable for vapor. Sci. Rep. 2019, 9, 6264. [Google Scholar] [CrossRef]
- Macchia, A.; Ruffolo, S.A.; Rivaroli, L.; Malagodi, M.; Licchelli, M.; Rovella, N.; Randazzo, L.; La Russa, M.F. Comparative study of protective coatings for the conservation of Urban Art. J. Cult. Herit. 2020, 41, 232–237. [Google Scholar] [CrossRef]
- Turk, J.; Mauko Pranjić, A.; Hursthouse, A.; Turner, R.; Hughes, J.J. Decision support criteria and the development of a decision support tool for the selection of conservation materials for the built cultural heritage. J. Cult. Herit. 2019, 37, 44–53. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.J. Syntheses of a novel nanomaterial for conservation of historic stones inspired by nature. Mater. Lett. 2007, 61, 4976–4979. [Google Scholar] [CrossRef]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stabil. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Doehne, E.F.; Price, C.A. Stone Conservation: An Overview of Current Research, 2nd ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2010; p. 158. [Google Scholar]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Predieri, G.; Barone, G.; Gemelli, G.; Mazzoleni, P.; Raneri, S.; Bersani, D.; Lottici, P.P. Nanocrystalline TiO2 coatings by sol–gel: Photocatalytic activity on Pietra di Noto biocalcarenite. J. Sol-Gel Sci. Technol. 2015, 75, 141–151. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Licciulli, A.; Calia, A.; Lettieri, M.; Diso, D.; Masieri, M.; Franza, S.; Amadelli, R.; Casarano, G. Photocatalytic TiO2 coatings on limestone. J. Sol-Gel Sci. Technol. 2011, 60, 437–444. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Huang, J.; Blake, D.M.; Maness, P.-C.; Huang, Z.; Fiest, J.; Jacoby, W.A. Photocatalytic Oxidation of Bacteria, Bacterial and Fungal Spores, and Model Biofilm Components to Carbon Dioxide on Titanium Dioxide-Coated Surfaces. Environ. Sci. Technol. 2002, 36, 3412–3419. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patra, R.; Majumdar, D.; Sen, K. Developing scenario of titania-based building materials for environmental remediation. Int. J. Environ. Sci. Technol. 2021, 18, 2077–2102. [Google Scholar] [CrossRef]
- Russell, H.S.; Frederickson, L.B.; Hertel, O.; Ellermann, T.; Jensen, S.S. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts 2021, 11, 675. [Google Scholar] [CrossRef]
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, A. Field study of air purifying paving elements containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- Yang, F.; Takahashi, Y.; Sakai, N.; Tatsuma, T. Visible light driven photocatalysts with oxidative energy storage abilities. J. Mater. Chem. 2011, 21, 2288–2293. [Google Scholar] [CrossRef]
- Du, Q.; Ma, J.; Shao, X.; Wang, W.; Tian, G. Core-shell structured TiO2@In2O3 for highly active visible-light photocatalysis. Chem. Phys. Lett. 2019, 714, 208–212. [Google Scholar] [CrossRef]
- Mas, N.; Hueso, J.L.; Martinez, G.; Madrid, A.; Mallada, R.; Ortega-Liebana, M.C.; Bueno-Alejo, C.; Santamaria, J. Laser-driven direct synthesis of carbon nanodots and application as sensitizers for visible-light photocatalysis. Carbon 2020, 156, 453–462. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Jin, J.; Wang, J.; Guo, J.; Yan, M.-H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D rare cubane-like tetramer Cu(II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130475. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Plank, J.; Ruiz-Izuriaga, D.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytically active coatings for cement and air lime mortars: Enhancement of the activity by incorporation of superplasticizers. Constr. Build. Mater. 2018, 162, 628–648. [Google Scholar] [CrossRef]
- Speziale, A.; González-Sánchez, J.F.; Taşcı, B.; Pastor, A.; Sánchez, L.; Fernández-Acevedo, C.; Oroz-Mateo, T.; Salazar, C.; Navarro-Blasco, I.; Fernández, J.M.; et al. Development of Multifunctional Coatings for Protecting Stones and Lime Mortars of the Architectural Heritage. Int. J. Archit. Herit. 2020, 14, 1008–1029. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, M.; Liu, Y.; Zheng, B.; Jiang, Q.; Lian, J. Structure and Photocatalysis of TiO2/ZnO Double-Layer Film Prepared by Pulsed Laser Deposition. Mater. Trans. 2012, 53, 463–468. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Varbanov, P.S.; Vocciante, M.; Fabiano, B. Bismuth oxide-related photocatalysts in green nanotechnology: A critical analysis. Front. Chem. Sci. Eng. 2018, 12, 878–892. [Google Scholar] [CrossRef]
- Rani, M.; Keshu Shanker, U. Efficient degradation of organic pollutants by novel titanium dioxide coupled bismuth oxide nanocomposite: Green synthesis, kinetics and photoactivity. J. Environ. Manag. 2021, 300, 113777. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Lee, S.H.; Hwang, I.N.; Kang, I.C.; Kim, M.S.; Kim, S.H.; Son, H.H.; Oh, W.M. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e96–e102. [Google Scholar] [CrossRef] [PubMed]
- Shajari-Ghasemkheili, S.; Sarraf-Mamoory, R. Development of a transparent silica-titania-methyl siliconate nanocoating with photocatalytic-hydrophobic properties aided by response surface method. Mater. Res. Express. 2019, 6, 106430. [Google Scholar] [CrossRef]
- Aldoasri, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Protecting of Marble Stone Facades of Historic Buildings Using Multifunctional TiO2 Nanocoatings. Sustainability 2017, 9, 2002. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Gobakis, K.; Kolokotsa, D.; Maravelaki-Kalaitzaki, N.; Perdikatsis, V.; Santamouris, M. Development and analysis of advanced inorganic coatings for buildings and urban structures. Energy Build. 2015, 89, 196–205. [Google Scholar] [CrossRef]
- Kamegawa, T.; Irikawa, K.; Yamashita, H. Unique Surface Properties of Nanocomposite Thin Film Photocatalysts of TiO2 and Poly(tetrafluoroethylene). Chem. Lett. 2015, 44, 509–511. [Google Scholar] [CrossRef]
- Kamegawa, T.; Shimizu, Y.; Yamashita, H. Superhydrophobic Surfaces with Photocatalytic Self-Cleaning Properties by Nanocomposite Coating of TiO2 and Polytetrafluoroethylene. Adv. Mater. 2012, 24, 3697–3700. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki, N.P. TiO2–SiO2–PDMS Nanocomposites with Self-Cleaning Properties for Stone Protection and Consolidation; Geological Society, London, Special Publications: London, UK, 2016; Volume 416, pp. 285–292. [Google Scholar]
- Lanka, S.; Alexandrova, E.; Kozhukhova, M.; Hasan, M.S.; Nosonovsky, M.; Sobolev, K. Tribological and Wetting Properties of TiO2 Based Hydrophobic Coatings for Ceramics. J. Tribol. 2019, 141, 101301. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for Cultural Heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Bico, J.; Teo, K.B.K.; Chhowalla, M.; Amaratunga, G.A.J.; Milne, W.I.; McKinley, G.H.; Gleason, K.K. Superhydrophobic Carbon Nanotube Forests. Nano Lett. 2003, 3, 1701–1705. [Google Scholar] [CrossRef]
- Meseck, G.R.; Käch, A.; Seeger, S. Three-Dimensional Organization of Surface-Bound Silicone Nanofilaments Revealed by Focused Ion Beam Nanotomography. J. Phys. Chem. C 2014, 118, 24967–24975. [Google Scholar] [CrossRef]
- Lee, C.G.; Na, K.H.; Kim, W.T.; Park, D.C.; Yang, W.H.; Choi, W.Y. TiO2/ZnO Nanofibers Prepared by Electrospinning and Their Photocatalytic Degradation of Methylene Blue Compared with TiO2 Nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, T.; Wang, L.; Zhang, S.; Zhang, G.; Xia, X. Hollow Microsphere TiO2/ZnO p–n Heterojuction with High Photocatalytic Performance for 2,4-Dinitropheno Mineralization. Nano 2017, 12, 1750076. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Šutka, A.; Järvekülg, M.; Gross, K.A. Photocatalytic Nanoheterostructures and Chemically Bonded Junctions Made by Solution-Based Approaches. Crit. Rev. Solid State Mat. Sci. 2019, 44, 239–263. [Google Scholar] [CrossRef]
- Thakur, S.; Maiti, S.; Pal, S.; Chattopadhyay, K.K. Geometrically Intricate Oxide-Based Heterostructure Over Flexible Platform: Morphology-Induced Catalytic Performance Enhancement Under UV Light. International Conference on Communication, Devices and Networking, ICCDN 2017. In Advances in Communication, Devices and Networking: Proceedings of ICCDN 2017; Sarkar, S.K., Bera, R., Chakraborty, S., Eds.; Springer: Berlin, Germany, 2018; pp. 21–27. [Google Scholar] [CrossRef]
- Ungan, H.; Tekin, T. Effect of the sonication and coating time on the photocatalytic degradation of TiO2, TiO2-Ag, and TiO2-ZnO thin film photocatalysts. Chem. Eng. Commun. 2020, 207, 896–903. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; González-Sánchez, J.F.; Fernández, J.M.; Sirera, R.; Navarro-Blasco, Í.; Alvarez, J.I. Influence of Two Polymer-Based Superplasticizers (Poly-naphthalene Sulfonate, PNS, and Lignosulfonate, LS) on Compressive and Flexural Strength, Freeze-Thaw, and Sulphate Attack Resistance of Lime-Metakaolin Grouts. Polymers 2018, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Yang Teoh, W.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Plank, J.; Vlad, D.; Brandl, A.; Chatziagorastou, P. Colloidal chemistry examination of the steric effect of polycarboxylate superplasticizers. Cem. Int. 2005, 3, 100–110. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Su, C. Effects of material properties on sedimentation and aggregation of titanium dioxide nanoparticles of anatase and rutile in the aqueous phase. J. Colloid Interface Sci. 2011, 363, 84–91. [Google Scholar] [CrossRef]
- Kong, H.J.; Bike, S.G.; Li, V.C. Electrosteric stabilization of concentrated cement suspensions imparted by a strong anionic polyelectrolyte and a non-ionic polymer. Cem. Concr. Res. 2006, 36, 842–850. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB value on oil-in-water emulsions: Droplet size, rheological behavior, zeta-potential, and creaming index. J. Ind. Eng. Chem. 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Yang, Y. Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants. Materials 2020, 13, 3514. [Google Scholar] [CrossRef]
- Tkachenko, N.H.; Yaremko, Z.M.; Bellmann, C.; Soltys, M.M. The influence of ionic and nonionic surfactants on aggregative stability and electrical surface properties of aqueous suspensions of titanium dioxide. J. Colloid Interface Sci. 2006, 299, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Edgar, M.; Melo, R. La rugosidad de las superficies: Topometría. Ingenierías 2001, 4, 27–33. [Google Scholar]
- UNE-EN 127197-1:2013; Application of the Test Method to Evaluate the Performance in Air Purification Using Photocatalytic Semiconductor Materials Embedded in Precast Concrete Products. Part 1: Elimination of Nitrogen Oxides. UNE-EN (Una Norma Española-European Norm): Madrid, Spain, 2013.
- Ballari, M.M.; Yu, Q.L.; Brouwers, H.J.H. Experimental study of the NO and NO2 degradation by photocatalytically active concrete. Catal. Today 2011, 1, 175–180. [Google Scholar] [CrossRef]
- Dylla, H.; Hassan, M.M.; Mohammad, L.N.; Rupnow, T.; Wright, E. Evaluation of environmental effectiveness of titanium dioxide photocatalyst coating for concrete pavement. Trans. Res. Rec. 2010, 2164, 46–51. [Google Scholar] [CrossRef]
- Cros, C.J.; Terpeluk, A.L.; Crain, N.E.; Juenger, M.C.G.; Corsi, R.L. Influence of environmental factors on removal of oxides of nitrogen by a photocatalytic coating. J. Air Waste Manag. Assoc. 2015, 65, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; Haq, A.U.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Balbuena, J.; Cruz-Yusta, M.; Sánchez, L.; Navarro-Blasco, I.; Fernández, J.; Alvarez, J. Photocatalytic NOx abatement by calcium aluminate cements modified with TiO2: Improved NO2 conversion. Cem. Concr. Res. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Navarro-Blasco, Í.; Fernández, J.M.; Duran, A.; Sirera, R.; Álvarez, J.I. A novel use of calcium aluminate cements for recycling waste foundry sand (WFS). Constr. Build. Mater. 2013, 48, 218–228. [Google Scholar] [CrossRef]
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. ISO (International Organization for Standardization): Geneva, Switzerland, 2016.
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef]
- Heikkilä, U.; Beer, J.; Feichter, J. Meridional transport and deposition of atmospheric 10Be. Atmos. Chem. Phys. 2009, 9, 515–527. [Google Scholar] [CrossRef]
- Prieto, C.; Lagaron, J.M. Nanodroplets of Docosahexaenoic Acid-Enriched Algae Oil Encapsulated within Microparticles of Hydrocolloids by Emulsion Electrospraying Assisted by Pressurized Gas. Nanomaterials 2020, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Fronzi, M.; Assadi, M.H.N.; Hanaor, D.A.H. Theoretical insights into the hydrophobicity of low index CeO2 surfaces. Appl. Surf. Sci. 2019, 478, 68–74. [Google Scholar] [CrossRef]
- Han, S.; Yang, R.; Li, C.; Yang, L. The Wettability and Numerical Model of Different Silicon Microstructural Surfaces. Appl. Sci. 2019, 9, 566. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. A Perfectly Hydrophobic Surface (θ A/θ R = 180°/180°). J. Am. Chem. Soc. 2006, 128, 9052–9053. [Google Scholar] [CrossRef]
- Sbeih, S.; Steffen, W.; Kappl, M. Anti-wettability of Chemically and Physically Modified Glass Surfaces. Adv. Mater. Proc. 2021, 6, 21010421. [Google Scholar] [CrossRef]
- González Sánchez, A. Obtención de Recubrimientos Superhidrofugantes Para Materiales de Construcción, Mediante el Uso de Nanopartículas de Sílice Funcionalizadas. Bachelor’s Thesis, UCA, Cádiz, Spain, 2016. Available online: https://rodin.uca.es/handle/10498/20685 (accessed on 25 March 2023).
- Fornasini, L.; Bergamonti, L.; Bondioli, F.; Bersani, D.; Lazzarini, L.; Paz, Y.; Lottici, P.P. Photocatalytic N-doped TiO2 for self-cleaning of limestones. Eur. Phys. J. Plus. 2019, 134, 539. [Google Scholar] [CrossRef]


















| SPHB | OHB | ||
|---|---|---|---|
| Dispersions | Average (mV) | Dispersions | Average (mV) |
| BiZn1 | −24.91 ± 1.22 | BiZn8 | −28.70 ± 0.87 |
| BiZn2 | −30.42 ± 0.91 | BiZn9 | −44.30 ± 0.87 |
| BiZn3 | −35.52 ± 1.45 | BiZn10 | −40.40 ± 1.52 |
| BiZn4 | −31.37 ± 1.42 | BiZn11 | −38.00 ± 0.49 |
| BiZn5 | −34.73 ± 1.39 | BiZn12 | −38.90 ± 0.41 |
| BiZn6 | −35.32 ± 2.11 | BiZn13 | −34.70 ± 1.36 |
| BiZn7 | −24.94 ± 1.01 | BiZn14 | −32.80 ± 0.41 |
| SPHB | OHB | ||
|---|---|---|---|
| Dispersions | Results (Microns) | Dispersions | Results (Microns) |
| BiZn1 granite | 31.00 ± 0.00 | BiZn8 granite | 37.00 ± 0.00 |
| BiZn1 limestone | 38.50 ± 0.71 | BiZn8 limestone | 34.00 ± 2.83 |
| BiZn1 sandstone | 38.00 ± 1.41 | BiZn8 sandstone | 34.00 ± 1.41 |
| BiZn2 granite | 31.00 ± 0.00 | BiZn9 granite | 37.50 ± 0.71 |
| BiZn2 limestone | 35.50 ± 0.71 | BiZn9 limestone | 36.00 ± 0.71 |
| BiZn2 sandstone | 33.50 ± 0.71 | BiZn9 sandstone | 34.50 ± 0.71 |
| BiZn3 granite | 32.50 ± 0.71 | BiZn10 granite | 35.50 ± 0.71 |
| BiZn3 limestone | 40.50 ± 0.71 | BiZn10 limestone | 33.50 ± 0.71 |
| BiZn3 sandstone | 37.50 ± 0.71 | BiZn10 sandstone | 35.50 ± 0.71 |
| BiZn4 granite | 37.50 ± 0.71 | BiZn11 granite | 32.50 ± 0.71 |
| BiZn4 limestone | 40.00 ± 1.41 | BiZn11 limestone | 28.50 ± 0.71 |
| BiZn4 sandstone | 39.50 ± 2.12 | BiZn11 sandstone | 47.50 ± 0.71 |
| BiZn5 granite | 38.00 ± 1.41 | BiZn12 granite | 27.50 ± 0.71 |
| BiZn5 limestone | 40.00 ± 2.83 | BiZn12 limestone | 33.50 ± 0.71 |
| BiZn5 sandstone | 39.00 ± 2.83 | BiZn12 sandstone | 34.00 ± 1.41 |
| BiZn6 granite | 39.50 ± 2.12 | BiZn13 granite | 26.50 ± 0.71 |
| BiZn6 limestone | 44.50 ± 0.71 | BiZn13 limestone | 25.00 ± 1.41 |
| BiZn6 sandstone | 40.50 ± 0.71 | BiZn13 sandstone | 34.50 ± 0.71 |
| BiZn7 granite | 37.50 ± 0.71 | BiZn14 granite | 26.50 ± 0.71 |
| BiZn7 limestone | 46.50 ± 2.12 | BiZn14 limestone | 26.50 ± 2.12 |
| BiZn7 sandstone | 41.00 ± 1.41 | BiZn14 sandstone | 45.50 ± 0.71 |
| SPHB | OHB | ||
|---|---|---|---|
| Dispersions | % Loss of Thickness | Dispersions | % Loss of Thickness |
| BiZn1 granite | 1.6 | BiZn8 granite | 20.3 |
| BiZn1 limestone | 1.3 | BiZn8 limestone | 11.8 |
| BiZn1 sandstone | 15.8 | BiZn8 sandstone | 13.2 |
| BiZn2 granite | 17.7 | BiZn9 granite | 22.7 |
| BiZn2 limestone | 7.0 | BiZn9 limestone | 11.1 |
| BiZn2 sandstone | 3.0 | BiZn9 sandstone | 17.4 |
| BiZn3 granite | 3.1 | BiZn10 granite | 15.5 |
| BiZn3 limestone | 4.9 | BiZn10 limestone | 22.4 |
| BiZn3 sandstone | 12.0 | BiZn10 sandstone | 21.1 |
| BiZn4 granite | 8.0 | BiZn11 granite | 16.9 |
| BiZn4 limestone | 5.0 | BiZn11 limestone | 0.0 |
| BiZn4 sandstone | 12.7 | BiZn11 sandstone | 32.6 |
| BiZn5 granite | 9.2 | BiZn12 granite | 3.6 |
| BiZn5 limestone | 8.8 | BiZn12 limestone | 20.9 |
| BiZn5 sandstone | 19.2 | BiZn12 sandstone | 10.3 |
| BiZn6 granite | 11.4 | BiZn13 granite | 3.7 |
| BiZn6 limestone | 16.9 | BiZn13 limestone | 6.0 |
| BiZn6 sandstone | 17.3 | BiZn13 sandstone | 26.1 |
| BiZn7 granite | 13.3 | BiZn14 granite | 0.0 |
| BiZn7 limestone | 30.1 | BiZn14 limestone | 5.7 |
| BiZn7 sandstone | 22.0 | BiZn14 sandstone | 35.2 |
| Hysteresis (Water) | Hysteresis (Water) | Hysteresis (Hexadecane) | |||
|---|---|---|---|---|---|
| BiZn1 granite | 4.47 | BiZn8 granite | 9.24 | BiZn8 granite | 6.45 |
| BiZn1 limestone | 5.81 | BiZn8 limestone | 1.58 | BiZn8 limestone | 0.16 |
| BiZn1 sandstone | 8.46 | BiZn8 sandstone | 1.74 | BiZn8 sandstone | 7.76 |
| BiZn2 granite | 0.42 | BiZn9 granite | 5.90 | BiZn9 granite | 10.27 |
| BiZn2 limestone | 5.61 | BiZn9 limestone | 0.52 | BiZn9 limestone | 0.70 |
| BiZn2 sandstone | 7.38 | BiZn9 sandstone | 11.00 | BiZn9 sandstone | 13.91 |
| BiZn3 granite | 3.96 | BiZn10 granite | 11.63 | BiZn10 granite | 2.66 |
| BiZn3 limestone | 1.50 | BiZn10 limestone | 0.68 | BiZn10 limestone | 0.25 |
| BiZn3 sandstone | 7.27 | BiZn10 sandstone | 7.88 | BiZn10 sandstone | 7.08 |
| BiZn4 granite | 2.15 | BiZn11 granite | 6.33 | BiZn11 granite | 6.01 |
| BiZn4 limestone | 2.99 | BiZn11 limestone | 0.69 | BiZn11 limestone | 6.17 |
| BiZn4 sandstone | 4.58 | BiZn11 sandstone | 6.82 | BiZn11 sandstone | 4.78 |
| BiZn5 granite | 0.15 | BiZn12 granite | 1.04 | BiZn12 granite | 1.64 |
| BiZn5 limestone | 3.50 | BiZn12 limestone | 5.59 | BiZn12 limestone | 1.29 |
| BiZn5 sandstone | 0.97 | BiZn12 sandstone | 1.34 | BiZn12 sandstone | 0.07 |
| BiZn6 granite | 11.69 | BiZn13 granite | 11.01 | BiZn13 granite | 3.96 |
| BiZn6 limestone | 4.84 | BiZn13 limestone | 5.14 | BiZn13 limestone | 1.66 |
| BiZn6 sandstone | 1.55 | BiZn13 sandstone | 1.01 | BiZn13 sandstone | 1.96 |
| BiZn7 granite | 0.10 | BiZn14 granite | 3.10 | BiZn14 granite | 7.40 |
| BiZn7 limestone | 3.08 | BiZn14 limestone | 3.37 | BiZn14 limestone | 5.55 |
| BiZn7 sandstone | 5.92 | BiZn14 sandstone | 9.30 | BiZn14 sandstone | 4.19 |
| SPHB Medium | ||||
| Coating | Dispersant | Photocatalyst (g) | Dispersant (mg) | SPHB (mL) |
| BiZn1 | - | 0.4 | 0 | 40 |
| BiZn2 | Tween20 | 0.4 | 3 | 40 |
| BiZn3 | Tween20 | 0.4 | 9 | 40 |
| BiZn4 | TritonX-100 | 0.4 | 3 | 40 |
| BiZn5 | TritonX-100 | 0.4 | 9 | 40 |
| BiZn6 | Brij35 | 0.4 | 3 | 40 |
| BiZn7 | Brij35 | 0.4 | 9 | 40 |
| OHB Medium | ||||
| Coating | Dispersant | Photocatalyst (g) | Dispersant (mg) | OHB (mL) |
| BiZn8 | - | 0.4 | 0 | 40 |
| BiZn9 | Tween20 | 0.4 | 3 | 40 |
| BiZn10 | Tween20 | 0.4 | 9 | 40 |
| BiZn11 | TritonX-100 | 0.4 | 3 | 40 |
| BiZn12 | TritonX-100 | 0.4 | 9 | 40 |
| BiZn13 | Brij35 | 0.4 | 3 | 40 |
| BiZn14 | Brij35 | 0.4 | 9 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tena-Santafé, V.M.; Fernández, J.M.; Fernández-Acevedo, C.; Oroz-Mateo, T.; Navarro-Blasco, Í.; Álvarez, J.I. Development of Photocatalytic Coatings for Building Materials with Bi2O3-ZnO Nanoparticles. Catalysts 2023, 13, 1412. https://doi.org/10.3390/catal13111412
Tena-Santafé VM, Fernández JM, Fernández-Acevedo C, Oroz-Mateo T, Navarro-Blasco Í, Álvarez JI. Development of Photocatalytic Coatings for Building Materials with Bi2O3-ZnO Nanoparticles. Catalysts. 2023; 13(11):1412. https://doi.org/10.3390/catal13111412
Chicago/Turabian StyleTena-Santafé, Víctor M., José M. Fernández, Claudio Fernández-Acevedo, Tamara Oroz-Mateo, Íñigo Navarro-Blasco, and José I. Álvarez. 2023. "Development of Photocatalytic Coatings for Building Materials with Bi2O3-ZnO Nanoparticles" Catalysts 13, no. 11: 1412. https://doi.org/10.3390/catal13111412
APA StyleTena-Santafé, V. M., Fernández, J. M., Fernández-Acevedo, C., Oroz-Mateo, T., Navarro-Blasco, Í., & Álvarez, J. I. (2023). Development of Photocatalytic Coatings for Building Materials with Bi2O3-ZnO Nanoparticles. Catalysts, 13(11), 1412. https://doi.org/10.3390/catal13111412