2.1. Morphology and Chemical Composition of the New Pt(poly), Pd(poly) and Rh(poly) Samples
Figure 1 displays SEM images and EDS spectra for the new samples Pt(poly) (a,b), Pd(poly) (c,d) and Rh(poly) (e,f) after purification of the surface by treatment in nitric acid and distilled water. The images were obtained in SE mode at
E0 = 20 keV and a ×1000 magnification. They show mostly a smooth surface of the samples with the rolling traces represented by hollows, protrusions and extended strips with a predominant size ≤ 1 µm. EDS spectra were recorded at a probe electron energy
E0 = 20 keV and the energy of X-ray quanta (
E) 0–10.0 keV on the regions with the size of 20 × 20 µm.
Figure 1b,d,f displays fragments of these EDS spectra in the energy range of 0–4.0 keV, which contains the main recorded X-ray peaks for C and O (
K series) and for Pt, Pd and Rh (
M and
L series). On Pt(poly), the X-ray peaks for Pt were obtained at
E = 1.59–2.69 keV (
M series) and
E > 8.27 keV (
L series), while the peaks for C and O were obtained at
E = 0.277 and 0.526 keV, as shown in
Figure 1b. For Pt(poly), the concentration of Pt, C and O appeared equal to 71.1, 28.2 and 0.7 at.%. On Pd(poly), the X-ray peaks were obtained for Pd at
E = 0.28–0.56 keV (
M series) and 2.5–3.55 keV (
L series), as well as the peak for C, as shown in
Figure 1d. The concentrations of Pd and C were equal to 82.1 and 17.9 at.%. On Rh(poly), the X-ray peaks for Rh were obtained at
E = 0.26–0.50 keV (
M series) and 2.38–3.36 keV (
L series), as shown in
Figure 1f. On Rh(poly), the concentrations of Rh, C and O constituted 62.1, 30.6 and 7.3 at.%. It should be noted that Fe impurities detected in the concentration from 0.1 to 0.3 at.% on some regions of this sample may belong to the initial metal.
According to EDS data, the new samples Pt(poly), Pd(poly) and Rh(poly) contain, in addition to metals, only C and O impurities; other impurities are not detected. It should be noted that the detection limit of elements for standard EDS analysis is ca. 0.1 wt.%. Therefore, it is impossible to detect the impurity elements that could be present in the metals according to GOST 13498-2010, 13462-2010 and 13098-2006 in the concentration ≤0.1 wt.%. In addition, according to these GOSTs, the above-listed impurities can be absent in the metals (see below
Section 4).
Note that at the temperature of the samples (ca. 1100 K) used in this study, metal atoms can migrate both over the surface and in bulk during the oxidation of metals and catalytic oxidation of NH3. Mobility of the atoms increases with an elevation of the temperature, thus accelerating the recrystallization processes, which include the disappearance of various defects, grain nucleation and growth, as well as a faceting of the grain surface with different faces. The mobility of metal atoms at a temperature T K is estimated from the T/Tmelt value, where Tmelt is the melting point of a metal. For Rh, Pt and Pd, Tmelt is equal to 2239, 2042 and 1825 K, respectively. It is commonly accepted that at T/Tmelt ≤ 0.3, metal atoms have a low mobility, which increases considerably when the Tamman temperature (T), for which T/Tmelt~0.5 or T/Tmelt > 0.5, is reached. It should be noted that the Tamman temperature for Rh, Pt and Pd has the values of 1119.5, 1021.0 and 912.5 K, respectively. Hence, at the temperature used in this study (ca. 1100 K), atoms of the indicated metals will be highly mobile.
2.2. Morphology and Chemical Composition of Pt(poly), Pd(poly) and Rh(poly) in the O2 Atmosphere
X-ray photoelectron spectroscopy (XPS), XRD, SEM and EDS methods allowed us to reveal an essential effect of the treatment temperature in the O
2 atmosphere on the morphology, microstructure and chemical composition of the surface and subsurface layers of Pt(poly) [
21]. The noticeable dissolution of oxygen in the platinum lattice and the formation of particles and/or crystals of bulk oxide like PtO
2(solid) were not detected; however, we observed the accumulation of oxygen atoms on the platinum surface and grain boundaries in the concentration of 5–10 at.%.
Figure 2a shows the concentrations of oxygen estimated by EDS after treatment of the samples in O
2 at temperatures from 600 to 1400 K for 3 h, for Pt(poly) (1) at
Po
2 = 2.1 × 10
4 Pa, and for Pd(poly) (2), Rh(poly) (3)—at
Po
2 = 10
5 Pa. The dependence for Pt(poly) (1) was reported earlier in [
21]. As the temperature is increased from 600 to 1400 K, a minor decrease in the oxygen concentration from 10.0 to 7.0 at.% is observed for Pt(poly), whereas for Pd(poly) and Rh(poly) this value grows significantly to 33.0 and 69.1 at.% at 1400 K, respectively. Low concentrations of O atoms for Pt(poly) are related to the absence of both the dissolution of O atoms in the Pt lattice and the formation of PtO
2 solid oxide particles, whereas the dissolution of O atoms in the lattice of Pd and Rh and the formation of particles and/or crystals of PdO and Rh
2O
3 solid oxides lead to high oxygen concentrations on these metals.
Figure 2b displays temperature dependencies of the equilibrium O
2 pressure for solid oxides PtO
2 (1), PdO (2) and Rh
2O
3 (3), which dissociate by the reactions PtO
2(solid) = Pt(solid) + O
2(gas), 2PdO(solid) = 2Pd(solid) + O
2(gas) and 2/3Rh
2O
3(solid) = 4/3Rh + O
2(gas). The dependencies in
Figure 2b take into account the values of equilibrium O
2 pressure measured upon dissociation of these oxides in the temperature range of 595–655, 717–797 and 770–930 K, respectively [
22]. Regions of parameters above each curve correspond to the values at which the corresponding solid oxide is stable and under them—the neat metal. The dissociation heat values (∆
dissH0(T)) of PtO
2, PdO and Rh
2O
3 oxides, corresponding to the dependencies in
Figure 2b, for the reactions listed above are equal to 222, 239 and 222 kJ/mol O
2, respectively [
22]. In the NH
3 oxidation reactor employed in this study, the pressure of the ammonia–air mixture is equal to 3.6 bar. At the NH
3 content of 10 vol.%, the O
2 pressure is 6.8 × 10
4 Pa. One can see in
Figure 2b that the equilibrium
Po
2 pressure grows from 10
–5 to 6.8 × 10
4 Pa as the temperature is increased from 500 to 900 K for PtO
2 (1), from 600 to 1133 K for PdO (2), and from 650 to 1400 K for Rh
2O
3 (3). In addition, it follows from dependencies 1–3 in
Figure 2b that at the O
2 pressure in the reactor (6.8 × 10
4 Pa) and the NH
3 oxidation temperature (ca. 1133 K) a stable state for Pt is the neat metal, while for Rh—the solid oxide Rh
2O
3. The dependence of equilibrium
Po
2 for PdO (
Figure 2b, curve 2) intersects the value of
Po
2 = 6.8 × 10
4 Pa at
T~1133 K. It means that under the NH
3 oxidation conditions, at
T ≤ 1133 K, the PdO solid oxide is stable, while at
T ≥ 1133 K—the metallic Pd. As a result, even a slight deviation of the reactor temperature from 1133 K may lead to the formation and/or decomposition of PdO oxide on Pd(poly).
Figure S1 (Supplementary Materials) displays the SEM images of the Pt(poly) surface after treatment in O
2 at 1100 K and
Po
2 = 1 bar for 3 h. At low magnifications (
Figure S1a,b), distinct grain boundaries separating grains with different brightness are detected on the surface. At high magnifications (
Figure S1c,d), a continuous layer of regular facets is observed on the grain surface. Parallel stepped crystal facets with a step height from 50 to 100–200 nm fill the entire surface of each grain. Flat layered facets ca. 10 nm in height are detected on the surface of such stepped facets (
Figure S1d). The microfaceted surface of Pt(poly) was also observed in our earlier study after annealing in the O
2 atmosphere at 1300 K [
21].
Figure 3 displays SEM images of the Pd(poly) (a–d) and Rh(poly) (e–h) surface after treatment of the samples in O
2 at 1100 K and
Po
2 = 1.0 bar for 3 h. For Pd(poly), a continuous nonuniform layer containing fragments of different shapes and sizes that are separated with cracks 0.1–1.0 µm in width is detected at low magnifications (
Figure 3a,b). The images in
Figure 3a,b most likely demonstrate the partially decomposed surface oxide layer. Taking into account that the oxygen concentration in Pd(poly) after annealing in O
2 at 1100 K is equal to 48.6 at.% (
Figure 2a), the oxide layer in
Figure 3a–d can be attributed to PdO oxide. At high magnifications, crystal particles of PdO oxide with the size of 50–200 nm are detected in the oxide layer (
Figure 3c). In the right and left parts of the image displayed in
Figure 3c, one can see particles with such a size, which was estimated using the dimension mark in the image. In
Figure 3c,d, a crack with a width of ca. 500 nm is observed in the oxide layer; it was used to estimate the thickness of the oxide layer at 100–200 nm. In addition, a crystal Pd facet with a height of ca. 100 nm is detected in this crack.
For Rh(poly), a continuous dark layer is detected at low magnifications; a vertical strip ca. 10 µm in width consisting of bright particles is seen at the center of this layer (
Figure 3e). Images in
Figure 3f,g show spherical particles with a size of 100–200 nm. One can see in
Figure 3h that these particles reside in a continuous layer comprising 25–50 nm particles. Taking into account that the oxygen concentration in Rh(poly) after annealing in O
2 at 1100 K is equal to 61.1 at.% (
Figure 2a), the surface layer in
Figure 3e–h can be attributed to Rh
2O
3 oxide. For Rh, Pt and Pd, the 1100 K value is close to the Tamman temperature (
T/
Tmelt~0.5) for these metals because the
T/
Tmelt value for them is 0.49, 0.54 and 0.6, thus indicating high mobility of atoms of these metals. As a result, recrystallization and oxidation processes, forming the oxide particles and crystal facets on the grain surface, proceed very intensely. According to
Figure 2a, at 1100 K in the O
2 atmosphere, the oxygen concentration on Pt(poly) is ca. 10.0 at.%, on Pd(poly)—48.6 at.%, and on Rh(poly)—61.1 at.%. On Pt(poly), O atoms are accumulated on such defects as grain boundaries and dislocations; the dissolution of oxygen in the metal lattice is not observed [
21]. On Pd(poly) and Rh(poly), O atoms both penetrate the defects and intercalate in the lattice of metals. These processes lead to the formation of particles and/or crystals of PdO and Rh
2O
3 oxides on the Pd and Rh surfaces. This is demonstrated by SEM images of the surface of samples after annealing in O
2 at 1100 K. For Pt(poly), the observed surface is typical of the recrystallized polycrystalline metallic sample (
Figure S1). For Pd(poly), one can see a cracked and partially decomposed 100–200 nm thick oxide layer of PdO particles and/or crystals with a size of 50–200 nm (
Figure 3a–d). For Rh(poly), a continuous oxide layer of Rh
2O
3 oxide particles with the size of 25–50 nm is observed, which contains large particles and/or oxide crystals with the size of 100–200 nm (
Figure 3e–h). These results are consistent with the data on equilibrium O
2 pressure for dissociating PtO
2, PdO and Rh
2O
3 oxides (
Figure 2b). For
Po
2 = 1 bar and
T = 1100 K used in the study, stable for Pt is the metallic state (
Figure 2b, curve 1). For Pd, the dependence of equilibrium
Po
2 for PdO (
Figure 2b, curve 2) intersects the value of
Po
2 = 1 bar at
T~1100 K, which indicates that the formation and decomposition of PdO solid oxide can occur under the indicated conditions. For Rh, under such conditions, the solid oxide Rh
2O
3 is stable (
Figure 2b, curve 3).
The data obtained on the oxidation of Pt(poly), Pd(poly) and Rh(poly) were used to elucidate the effect exerted by the oxidation of metal on its etching initiated by NH3 oxidation with air oxygen. According to SEM and EDS data, after NH3 oxidation with air oxygen at T = 1133 K and the reactor pressure of 3.6 bar for 1, 5 and 10 h, etched structures of different chemical compositions appear on the surface of Pt(poly), Pd(poly) and Rh(poly). The structures detected after NH3 oxidation strongly differs from those observed after annealing in O2.
2.3. Morphology and Chemical Composition of Pt(poly), Pd(poly) and Rh(poly) after NH3 Oxidation with Air at 1133 K for 1, 5 and 10 Hours
Pt(poly) after NH3 oxidation. Figure 4 displays SEM images of the Pt(poly) surface after NH
3 oxidation with air oxygen at
T = 1133 K for 1 (a–d) and 5 (e–h) hours. Images obtained at low magnifications (
Figure 4a,b,e,f) show considerable changes in the surface morphology compared to the new Pt(poly) sample (
Figure 1a) and the sample after annealing in O
2 (
Figure S1). After NH
3 oxidation for 1 h, a granular structure with ca. 50–100 µm bright and dark grains separated by distinct grain boundaries is observed on the Pt(poly) surface (
Figure 4a,b). The surface of the bright grains contains regular parallel grooves (facets), which are similarly separated. In contrast, the surface of dark grains contains irregular facets and hollows, which have smaller sizes and lower concentrations than the facets on bright grains (
Figure 4c,d). Images obtained at high magnifications (
Figure 4c,d) demonstrate significant differences in the etching structures on adjacent grains. In the left part of images in
Figure 4c,d, each grain contains facets with a close orientation, which differs from the orientation of the facets on adjacent grains. Crystal facets in
Figure 4c,d have a step height of up to 0.5–1.0 µm. Differences in the etching structures of adjacent grains may be related to the different surface orientations of these grains, which can substantially affect the faceting during NH
3 oxidation.
After NH
3 oxidation for 5 h, a granular structure with a grain size of 50–100 µm is also detected on the Pt(poly) surface (
Figure 4e,f). The grain surface has close brightness; each grain contains etched structures represented by extended, parallel and similarly separated facets. Images obtained at high magnifications show a distinct grain boundary and large crystal facets with a step height of up to 1–2 µm (
Figure 4g,h). This sample was used in NH
3 oxidation for an additional 5 h. After NH
3 oxidation for 10 h, distinct grain boundaries, which separate grains with parallel facets having a step height of up to 1–2 µm, are seen on the sample surface. The revealed structure is close to that obtained after the oxidation for 5 h (
Figure 4e–h).
Thus, after NH
3 oxidation on Pt(poly) at 1133 K for 1, 5 and 10 h, the microgranular structure of metallic platinum with a grain size of 50–100 µm is detected on the surface. The grain surface predominantly contains regular, similarly separated crystal facets. In all cases, particles and/or crystals of such platinum oxides as PtO
2 were not observed. This is consistent with the temperature dependence of equilibrium O
2 pressure above PtO
2(solid), which testifies to the stable metallic state of Pt under the conditions of NH
3 oxidation used in this study (
Figure 2b). In addition, a comparison of images of the Pt(poly) surface after annealing in O
2 (
Figure S1) and after NH
3 oxidation (
Figure 4) at a temperature of ca. 1100 K shows their close microstructures. In both cases, the microgranular structure of metallic platinum with the faceted grain surface was observed; however, after annealing in O
2, the height of facets is 100–200 nm, while after NH
3 oxidation, facets with a size up to 1–2 µm are detected. This indicates that the etching of the Pt(poly) surface is more intense during the catalytic oxidation of NH
3 as compared to annealing in O
2. In [
11], after NH
3 oxidation for 386 h, both the terraces 5–10 µm in width with ca. 1 µm high facets, and crystal “cauliflowers” with the size of 5–20 µm were found on the surface of platinum wire.
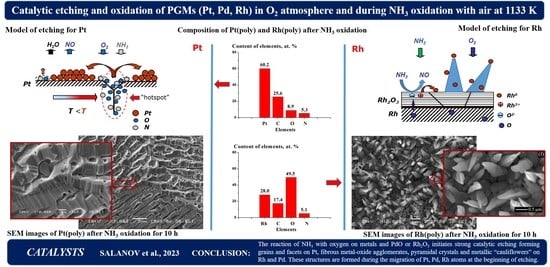
The concentration of elements on Pt(poly) after NH
3 oxidation was estimated from the EDS data obtained by the accumulation of EDS spectra for 150 s in three square regions of the surface with the size of 5 × 5 µm, which are located on different grains. The EDS spectra were recorded on the surface regions similar to those displayed in
Figure 4c,g. Pt, C, O and N were detected on all surface regions for all Pt(poly) samples. In addition, it should be noted that on some regions Fe, Si, Al and Mg impurities were detected in a low concentration, ≤0.1–0.5 at.%. Concentrations of these elements strongly differ on different regions and may be associated with the impurities that were present in the initial material or transferred to the catalyst from the gas flow and reactor during the oxidation of NH
3. Note also that concentrations of these elements were measured with a relatively high statistical error at the detection limit of this method. As a result, the indicated impurities may have low concentrations or be completely absent. Taking into account the indicated data, Fe, Si, Al and Mg elements were excluded from the list of detected elements, and only the main Pt, C, O and N elements were considered.
Figure 5 displays data on the chemical composition of the Pt(poly) (a–c) and Rh(poly) (d–f) after NH
3 oxidation at 1133 K for 1 (a,d), 5 (b,e) and 10 (c,f) hours according to EDS data. The concentrations of Pt, C, O and N in the three squares differed insignificantly from each other, so they were used to calculate the average concentrations.
Figure 5 indicates the obtained averaged concentrations of these elements after NH
3 oxidation for 1 (a), 5 (b) and 10 (c) hours. After NH
3 oxidation for 1 h, the concentrations of Pt, C, O and N were equal to 60.7, 29.3, 5.7 and 4.3 at.%, respectively (
Figure 5a). It should be noted that the obtained oxygen concentration (5.7 at.%) was close to that obtained after annealing of Pt(poly) in O
2 at 1133 K (ca. 10.0 at.%, as shown in
Figure 2a). It should also be noted that C and O were detected on some regions of the new Pt(poly) sample in the concentration of ca. 28.2 and 0.7 at.%, respectively. The detected carbon may include C atoms as the absorbed C
ab atoms on such defects as dislocations and grain boundaries in subsurface layers of the sample. Most likely, O and N atoms detected by EDS include predominantly the O
ab and N
ab atoms that were absorbed on defects in subsurface platinum layers. As the NH
3 oxidation time is extended from 1 to 5 and 10 h, a gradual decrease in the concentration of C (29.3, 26.6, 21.0 at.%) and its growth for O (5.7, 10.1, 10.9 at.%) are observed. The N concentration remains virtually constant, equal to 4.3, 6.4 and 5.2 at.%. Changes in the concentration of detected C and O with the extension of the NH
3 oxidation time testify to a gradual removal of C
ab from the subsurface region and accumulation of O
ab atoms in this region.
Rh(poly) after NH
3 oxidation.
Figure 6 displays images of the Rh(poly) surface after NH
3 oxidation with air oxygen at
T = 1133 K for 1 (a–d) and 5 (e–h) hours. Images obtained at low magnifications (
Figure 6a,e) demonstrate considerable changes in the surface morphology after NH
3 oxidation compared to the new Rh(poly) (
Figure 1e), and the sample annealed in the O
2 atmosphere, as shown in
Figure 3e–h. After NH
3 oxidation for 1 h, bright agglomerates, ca. 1 µm in diameter and extended agglomerates, ca. 1–2 µm in width and up to 30 µm in length, are observed on the Rh(poly) surface, as shown in
Figure 6a,b. Images obtained at high magnifications (
Figure 6c,d) show a continuous dark layer comprising particles with the size of ca. 0.5–1.0 µm, on which bright agglomerates with the fibrous structure reside. Fibers detected at high magnifications have a diameter of 25–50 nm. The revealed etching structure on the Rh(poly) surface, which was formed during the oxidation of NH
3 for 1 h, is determined by a strong effect exerted by the oxidation of rhodium on its etching under the indicated conditions. According to the dependence of equilibrium O
2 pressure above Rh
2O
3 oxide, at the O
2 pressure used in the study and
T ≤ 1400 K, the Rh
2O
3 oxide is stable, as shown in
Figure 2b, curve 3. In addition, after the oxidation of Rh(poly) in O
2 at 1100 K, a continuous Rh
2O
3 oxide layer consisting of particles with the size of 25–50 nm was observed. Spherical crystal particles with the size of 100–200 nm were detected in this layer, as shown in
Figure 3e–h. These data allow a conclusion that the NH
3 oxidation on Rh(poly) at 1133 K for 1 h leads to the formation of a continuous surface oxide layer consisting of particles and/or crystals of Rh
2O
3 oxide with the size of 0.5–1.0 µm, as shown in
Figure 6d. During the oxidation of NH
3 molecules, metal-oxide nanofibers and nanocrystals with the size of 25–50 nm emerge on the oxide layer. They gradually form metal-oxide fibrous agglomerates with a size of ca. 1 µm and agglomerates with a length of up to 30 µm, as shown in
Figure 6a–d. It should be noted that in [
12], after NH
3 oxidation for 23 h, “wool”-like and whisker deposits of Rh
2O
3 and “cauliflowers” with the size of 3–80 µm were detected on the surface of rhodium wire.
After NH
3 oxidation on Rh(poly) for 5 h, a layer of bright particles with a size below 1 µm is observed on the sample surface, as shown in
Figure 6e,f. Images obtained at high magnification show a continuous layer of pyramidal crystals oriented from the surface, as shown in
Figure 6g,h. In the initial step of NH
3 oxidation (
t = 1 h), a continuous Rh
2O
3 oxide layer is formed on the Rh(poly) surface. During the further oxidation of NH
3 (
t = 5 h), pyramidal crystals emerge on the surface of this oxide and gradually fill the entire surface. The crystals have a length of 1–2 µm, and their sizes are equal to 0.3–0.5 µm at the pyramid’s base and 0.05–0.1 µm at its vertex, as shown in
Figure 6h. After NH
3 oxidation on Rh(poly) for 10 h, a continuous etched layer of pyramidal crystals appears on the surface; the crystals are 1–2 µm in length, and their sizes are equal to 0.4–0.5 µm at the base of the pyramid and 0.1–0.2 µm at their vertices.
Figure 5d f illustrate data on the chemical composition of the Rh(poly) subsurface layer after NH
3 oxidation for 1 (d), 5 (e) and 10 (f) hours. The concentrations of the main elements and impurities were estimated similarly as for Pt(poly) (see above) from the EDS data obtained by the accumulation of EDS spectra on the surface regions with the size of 15 × 15 µm. Rh, C, O and N were detected on all surface regions for all Rh(poly) samples.
Figure 5d f indicates the averaged concentrations of these elements. After NH
3 oxidation for 1 h, the concentrations of Rh, C, O and N equal 29.2, 18.0, 48.2 and 4.6 at.%, respectively, as shown in
Figure 5d. It should be noted that the measured O concentration (48.2 at.%) is close to the value obtained after the oxidation of Rh(poly) in O
2 at 1100 K (61.1 at.%, as shown in
Figure 2a) but considerably exceeds the O concentration on the new sample (ca. 7.3 at.%). The obtained oxygen concentration testifies to the formation of Rh
2O
3 oxide during the oxidation of NH
3. The detected carbon may include C atoms residing on the surface and in subsurface layers of the sample. The detected oxygen corresponds mostly to O
2– ions of Rh
2O
3 oxide and nitrogen—to N
ab atoms absorbed on defects in the subsurface layer of the catalyst. An extension of the NH
3 oxidation time on Rh(poly) (1, 5 and 10 h) produces slight changes in the concentrations of elements: an increase for O (48.2, 49.0 and 51.3 at.%), a decrease for C (18.0, 19.2 and 15.0 at.%), and minor deviations for N (4.6, 5.9 and 4.9 at.%) and Rh (29.2, 25.9 and 28.8 at.%), as shown in
Figure 5d f. The concentrations of elements on Rh(poly) samples measured after NH
3 oxidation for 1, 5 and 10 h indicate that the oxidation of NH
3 proceeds on the surface of the Rh
2O
3 oxide layer for the entire period.
Pd(poly) after NH
3 oxidation.
Figure 7 displays SEM images of the Pd(poly) surface after NH
3 oxidation with air oxygen at
T = 1133 K for 1 (a–d) and 5 (e–h) hours. Images obtained at low magnifications (
Figure 7a,e) show essential changes in the surface morphology compared to the new Pd(poly) (
Figure 1c) and the sample annealed in O
2 (
Figure 3a−d). After NH
3 oxidation for 1 h, we observed on the Pd(poly) surface a continuous layer of agglomerates with a diameter of 1–5 µm and extended agglomerates having a width of ca. 1–5 µm and a length from 5 to 30 µm, as shown in
Figure 7a,b. SEM images obtained at high magnifications (
Figure 7c,d) demonstrate these agglomerates’ loose, fibrous surface structure. Fibers detected at high magnifications have a thickness in the range of 25–50 nm. The etched structure revealed on the Pd(poly) surface is caused by a considerable effect of Pd oxidation on its etching under the conditions of NH
3 oxidation. First of all, it should be noted that during the oxidation of NH
3 for 1 h the catalyst temperature may strongly deviate from the maintained value of ca. 1133 K. Taking into account a short period of NH
3 oxidation in this experiment (
t = 1 h), low values of the catalyst temperature (<1133 K) can be expected. Thus, after feeding the reaction mixture into the reactor, a ca. 1133 K temperature on the gauzes is reached approximately for 5 min during the exothermic oxidation of NH
3. It seems that the Pd(poly) sample is heated to this temperature much more slowly because the temperature mode is generally maintained by the exothermic oxidation of NH
3 on catalytic gauzes. After 1 h of NH
3 oxidation, feeding of NH
3 and, accordingly, heating of the catalyst and reactor was stopped, and the catalyst was gradually cooled to ca. 300 K for ca. 30 min. Taking into account the oxidation time of NH
3 as well as the estimated time of catalyst heating and cooling, one can suppose that the temperature of Pd(poly) sample could be lower than 1133 K for a substantial part of this period. According to
Figure 2b, curve 2, at
T < 1133 K and O
2 pressure used in the study, the PdO oxide is stable. In addition, after annealing of Pd(poly) in O
2 at 1100 K, a 100–200 nm thick nonuniform surface layer consisting of PdO particles with the size of 50–200 nm was found (
Figure 3a–d). The reaction of NH
3 molecules with the oxygen of the oxide layer leads to the emergence of metal-oxide nanofibers and nanocrystals with the size of 25–50 nm, which gradually form metal-oxide fibrous agglomerates ca. 1–5 µm in size and agglomerates with a length up to 5–30 µm (
Figure 7a–d). It should be noted that in [
13] NH
3 oxidation for 54 h resulted in the formation of terraces, facets, “cauliflowers”, “wool”-like deposits of PdO, and a continuous PdO oxide layer with a thickness of ca. 4 µm on the surface of palladium wire.
After NH
3 oxidation for 5 h, a continuous etched layer comprising crystals and porous crystal “cauliflowers” with different shapes and sizes of 10–30 µm is observed on the Pd(poly) surface, as shown in
Figure 7e,f. Such “cauliflowers” contain pores with a diameter 1–2 µm and are separated by extended voids 1–10 µm in width, as shown in
Figure 7g,h. After NH
3 oxidation for 54 h, palladium “cauliflowers” with the size of 4–55 µm were detected on the surface of palladium wire [
13]. In addition, images of crystal “cauliflowers” in
Figure 7e–h are close to the earlier obtained images of metallic “cauliflowers” on Pt–Pd–Rh−Ru gauzes after NH
3 oxidation at 1133 K for 50 h [
16,
17]. Taking into account this information, the etched layer observed on the Pd(poly) surface (
Figure 7e–h) indicates the formation of palladium “cauliflowers” during the oxidation of NH
3 at 1133 K for 5 h. Heating of the catalyst after feeding the NH
3 flow and cooling of the catalyst after turning off the flow were performed for ca. 5 and 30 min, respectively. This constitutes an insignificant part of this experiment’s entire period of NH
3 oxidation (
t = 5 h). As a result, upon NH
3 oxidation for 5 h, the Pd(poly) sample is kept mostly at
T~1133 K. In addition, the catalyst temperature during NH
3 oxidation may deviate to both sides from the maintained value (ca. 1133 K). Under the indicated conditions, the formation-decomposition of PdO oxide can proceed on Pd(poly) because, at the
Po
2 value used in this study and
T < 1133 K, the PdO oxide is stable, whereas at
T > 1133 K—the metallic palladium (
Figure 2b, curve 2). In addition, the reaction of NH
3 with the oxygen of PdO oxide will lead to the decomposition of the oxide particles. These processes will result in a gradual formation of palladium “cauliflowers”. As a result, after 5 h of NH
3 oxidation at
T~1133 K, a continuous etched layer of “cauliflowers” is observed on Pd(poly) (
Figure 7e–h). It should be noted that after 5 h of NH
3 oxidation, deep cracks destroying the sample appeared on Pd(poly); these cracks made it impossible to mount the sample in the reactor and perform NH
3 oxidation for another 5 h. In [
13], after NH
3 oxidation for 54 h, the destruction of palladium wire was also observed.
Figure S2 (Supplementary Material) displays data on the chemical composition of the Pd(poly) subsurface layer after NH
3 oxidation for 1 (a) and 5 (b) hours. The concentrations of the main elements and impurities were estimated in a similar manner as for Pt(poly) (see above) from the EDS data obtained by the accumulation of EDS spectra on the surface regions with the size of 35 × 35 µm after NH
3 oxidation for 1 h (
Figure 7a) and 5 × 5 µm—for 5 h (
Figure 7f). Pd, C, O and N were detected on all surface regions for all Pd(poly) samples.
In
Figure S2, one can see the averaged concentrations of these elements. After NH
3 oxidation for 1 h, the concentrations of Pd, C, O and N were equal to 51.7, 4.1, 39.2 and 5.0 at.%, respectively, as shown in
Figure S2a. Note that the observed O concentration (39.2 at.%) is close to the value obtained after annealing of Pd(poly) in O
2 at 1100 K (48.6 at.%,
Figure 2a) but considerably exceeds the O concentration (ca. 0.0 at.%) measured on the new sample. The detected carbon may be associated with C impurities in subsurface Pd layers. The detected nitrogen may correspond mostly to the N
ab atoms absorbed on defects in the subsurface layers of the catalyst. An extension of the NH
3 oxidation time from 1 to 5 h is accompanied by a significant decrease in the concentration of O (from 39.2 to 2.0 at.%) and C (from 4.1 to 2.3 at.%) and noticeable growth of the N content (from 5.0 to 11.3 at.%), as shown in
Figure S2a,b. Changes in the surface morphology and O concentration on Pd(poly) that occur with extension of the NH
3 oxidation time indicate that the oxidation of NH
3 proceeds mostly on PdO oxide at the very onset of the process (at
t = 1 h) and later on palladium “cauliflowers” (at
t = 5 h).