Role and Application of Biocatalysts in Cancer Drug Discovery
Abstract
:1. Introduction
2. Role of Biocatalyst in Tumor Tissue
2.1. MMPs as Biocatalyst
2.2. Polysaccharides
2.3. Prostate-Specific Antigen
2.4. Indoleamine 2,3-Dioxygenase as Biocatalyst
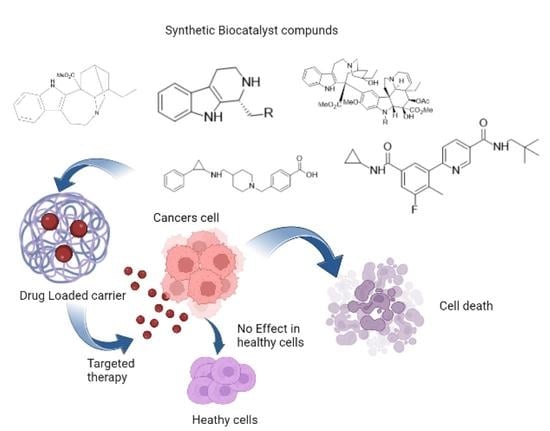
3. Biocatalyst in Natural Product Synthesis
4. Biocatalysts Used in Nanoparticles for Tumor-Targeted Delivery
4.1. Site-Specific Drug Delivery
4.2. Enzyme-Responsive Drug Delivery System
4.3. Photothermal Therapy
5. Tumor Microenvironment and the Role of Biocatalyst in Its Improvement
5.1. Tumor Microenvironment and Other Cells
5.2. Macrophages and Myeloid Suppressor Cells
5.3. Non-Malignant Cells
5.4. Bioenzymes and Their Role in Carrying Biocatalysts as Nanomedicines
5.5. Biocatalyst Used for In Vitro/In Vivo Studies in Cancer Therapy
5.6. Enzyme-Based Nanomedicines for Tumor Microenvironment
5.7. Magnetic Nanocatalysts in TME
5.8. Glucose-Oxidase-Based Enzyme-Catalyzed Technique
5.9. Other Biocatalytic Reactions
6. Biocatalyst Used for Synthesis of Different Compounds
7. Summary and Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, S.; Cowan, D.; Woodley, J. The search for the ideal biocatalyst. Nat. Biotechnol. 2002, 20, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Mansi, K.; Kumar, R.; Kaur, J.; Mehta, S.; Pandey, S.K.; Kumar, D.; Dash, A.K.; Gupta, N. DL-Valine assisted fabrication of quercetin loaded CuO nanoleaves through microwave irradiation method: Augmentation in its catalytic and antimicrobial efficiencies. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100306. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Prim. 2021, 1, 46. [Google Scholar] [CrossRef]
- Alcántara, A.R. Biocatalysis and Pharmaceuticals: A Smart Tool for Sustainable Development. Catalysts 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Ding, Y. Recent advances in biocatalyst discovery, development and applications. Bioorganic Med. Chem. 2014, 22, 5604–5612. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Nanoscale biocatalyst systems. Curr. Opin. Biotechnol. 2006, 17, 574–579. [Google Scholar] [CrossRef]
- Turner, N.J.; Humphreys, L. Biocatalysis in Organic Synthesis: The Retrosynthesis Approach; RSC: London, UK, 2018. [Google Scholar]
- Wahler, D.; Reymond, J.-L. Novel methods for biocatalyst screening. Curr. Opin. Chem. Biol. 2001, 5, 152–158. [Google Scholar] [CrossRef]
- Almonacid, D.E.; Yera, E.R.; Mitchell, J.B.; Babbitt, P.C. Quantitative comparison of catalytic mechanisms and overall reactions in convergently evolved enzymes: Implications for classification of enzyme function. PLoS Comput. Biol. 2010, 6, e1000700. [Google Scholar] [CrossRef] [Green Version]
- Silverman, R.B. Organic Chemistry of Enzyme-Catalyzed Reactions; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Es, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Dash, A.K.; Mukherjee, D.; Dhulap, A.; Haider, S.; Kumar, D. Green chemistry appended synthesis, metabolic stability and pharmacokinetic assessment of medicinally important chromene dihydropyrimidinones. Bioorganic Med. Chem. Lett. 2019, 29, 126750. [Google Scholar] [CrossRef]
- Li, M.; Qin, J.; Xiong, K.; Jiang, B.; Zhang, T. Review of arginase as a promising biocatalyst: Characteristics, preparation, applications and future challenges. Crit. Rev. Biotechnol. 2021, 42, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Feng, J.; Song, S.; Liu, K.; Du, K.; Zhou, Y.; Lv, K.; Zhang, H. Tumor-targeted biocatalyst with self-accelerated cascade reactions for enhanced synergistic starvation and photodynamic therapy. Nano Today 2022, 43, 101433. [Google Scholar] [CrossRef]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhu, B.; Deng, Y.; Li, T.; Tian, Q.; Yuan, Z.; Ma, L.; Cheng, C.; Guo, Q.; Qiu, L. Biocatalytic and Antioxidant Nanostructures for ROS Scavenging and Biotherapeutics. Adv. Funct. Mater. 2021, 31, 2101804. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.; Zhang, M.; Yu, W.; Gao, F.; Li, C.; Wang, S.-B.; Feng, J.; Zhang, X.-Z. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. ACS Nano 2018, 12, 12181–12192. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Li, G.; Wu, S. Functional Nanoparticles for Enhanced Cancer Therapy. Pharmaceutics 2022, 14, 1682. [Google Scholar] [CrossRef]
- Pellikainen, J.M.; Ropponen, K.M.; Kataja, V.V.; Kellokoski, J.K.; Eskelinen, M.J.; Kosma, V.-M. Expression of Matrix Metalloproteinase (MMP)-2 and MMP-9 in Breast Cancer with a Special Reference to Activator Protein-2, HER2, and Prognosis. Clin. Cancer Res. 2004, 10, 7621–7628. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-C.; Cao, D.-C.; Liu, Y.; Hou, Y.-F.; Wu, J.; Lu, J.-S.; Di, G.-H.; Liu, G.; Li, F.-M.; Ou, Z.-L.; et al. Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res. Treat. 2004, 88, 75–85. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin Inhibits Migration and Invasion of MDA-MB-231 and 4T1 Breast Cancer Cells by Suppressing MMP-2 and MMP-9 Through PI3K/AKT Signaling Pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef]
- Ji, T.; Li, S.; Zhang, Y.; Lang, J.; Ding, Y.; Zhao, X.; Zhao, R.; Li, Y.; Shi, J.; Hao, J.; et al. An MMP-2 Responsive Liposome Integrating Antifibrosis and Chemotherapeutic Drugs for Enhanced Drug Perfusion and Efficacy in Pancreatic Cancer. ACS Appl. Mater. Interfaces 2016, 8, 3438–3445. [Google Scholar] [CrossRef]
- Renoux, B.; Raes, F.; Legigan, T.; Péraudeau, E.; Eddhif, B.; Poinot, P.; Tranoy-Opalinski, I.; Alsarraf, J.; Koniev, O.; Kolodych, S.; et al. Targeting the tumour microenvironment with an enzyme-responsive drug delivery system for the efficient therapy of breast and pancreatic cancers. Chem. Sci. 2017, 8, 3427–3433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Naveed, M.; Azeem, I.; Faisal, A.; Nazar, M.F.; Yameen, B. Colon specific enzyme responsive oligoester crosslinked dextran nanoparticles for controlled release of 5-fluorouracil. Int. J. Pharm. 2020, 586, 119605. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Jin, W.; Cheng, K. Prostate cancer relevant antigens and enzymes for targeted drug delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef] [Green Version]
- Lövgren, J.; Rajakoski, K.; Karp, M.; Lundwall, Å.; Lilja, H. Activation of the Zymogen Form of Prostate-Specific Antigen by Human Glandular Kallikrein 2. Biochem. Biophys. Res. Commun. 1997, 238, 549–555. [Google Scholar] [CrossRef]
- Balk, S.P.; Ko, Y.-J.; Bubley, G.J. Biology of Prostate-Specific Antigen. J. Clin. Oncol. 2003, 21, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Cary, K.C.; Cooperberg, M.R. Biomarkers in prostate cancer surveillance and screening: Past, present, and future. Ther. Adv. Urol. 2013, 5, 318–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.M.; Sloane, B.F. multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef]
- Tan, G.-J.; Peng, Z.-K.; Lu, J.-P.; Tang, F.-Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 2013, 4, 91–101. [Google Scholar] [CrossRef]
- Palermo, C.; Joyce, J.A. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol. Sci. 2008, 29, 22–28. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tonk, R.; Shekhar, R.; Dohare, S.; Kumar, D. Need to focus on inhibitory activity of benzimidazole analogues against indolamine 2,3-dioxygenase-1 (IDO-1). EXCLI J. 2022, 21, 904–905. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Wu, W.; Ling, D.; Zhang, Q.; Zhao, P.; Hu, X. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials 2021, 276, 121018. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, T.; Qindeel, M.; Asim.Ur.Rehman; Tarhini, M.; Ahmed, N.; Elaissari, A. Exploiting proteases for cancer theranostic through molecular imaging and drug delivery. Int. J. Pharm. 2020, 587, 119712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Thodey, K.; Trenchard, I.; Cravens, A.; Smolke, C.D. Complete biosynthesis of noscapine and halogenated alkaloids in yeast. Proc. Natl. Acad. Sci. USA 2018, 115, E3922–E3931. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Mustafa, N.R.; Tang, K.; Choi, Y.H.; Verpoorte, R. Monoterpenoid indole alkaloids biosynthesis and its regulation in Catharanthus roseus: A literature review from genes to metabolites. Phytochem. Rev. 2015, 15, 221–250. [Google Scholar] [CrossRef]
- Yi, D.; Bayer, T.; Badenhorst, C.P.S.; Wu, S.; Doerr, M.; Höhne, M.; Bornscheuer, U.T. Recent trends in biocatalysis. Chem. Soc. Rev. 2021, 50, 8003–8049. [Google Scholar] [CrossRef]
- Teufel, R.; Kaysser, L.; Villaume, M.T.; Diethelm, S.; Carbullido, M.K.; Baran, P.S.; Moore, B.S. One-Pot Enzymatic Synthesis of Merochlorin A and B. Angew. Chem. Int. Ed. 2014, 53, 11019–11022. [Google Scholar] [CrossRef] [Green Version]
- Ashfaq, U.A.; Riaz, M.; Yasmeen, E.; Yousaf, M.Z. Recent Advances in Nanoparticle-Based Targeted Drug-Delivery Systems against Cancer and Role of Tumor Microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 317–353. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Stoyanova, E.; Mitova, V.; Shestakova, P.; Momekov, G.; Momekova, D.; Koseva, N. Star-shaped nano-conjugates of cisplatin with high drug payload. Int. J. Pharm. 2011, 404, 220–230. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the core. J. Control. Release 2001, 74, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Suwa, S.; Kataoka, K. Introduction of cisplatin into polymeric micelle. J. Control. Release 1996, 39, 351–356. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Su, W.-Y.; Yen, K.-C.; Yang, K.-C.; Lin, F.-H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shambhwani, D.; Pandey, S.; Singh, J.; Lalhlenmawia, H.; Kumarasamy, M.; Singh, S.K.; Chellappan, D.K.; Gupta, G.; Prasher, P.; et al. Advances in Lung Cancer Treatment Using Nanomedicines. ACS Omega 2022, 8, 10–41. [Google Scholar] [CrossRef]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Nezhad-Mokhtari, P.; Arsalani, N.; Ghorbani, M.; Hamishehkar, H. Development of biocompatible fluorescent gelatin nanocarriers for cell imaging and anticancer drug targeting. J. Mater. Sci. 2018, 53, 10679–10691. [Google Scholar] [CrossRef]
- Farooq, M.A.; Aquib; Farooq, A.; Khan, D.H.; Maviah, M.B.J.; Filli, M.S.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A.; et al. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: An overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef] [Green Version]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mater. Sci. Eng. C 2021, 123, 112027. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [Green Version]
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of Human Hyaluronidase-1, a Hyaluronan Hydrolyzing Enzyme Involved in Tumor Growth and Angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Zuo, T.; Ma, S.; Xokrat, N.; Hu, Z.; Wang, Z.; Xu, R.; Wei, Y.; Shen, Q. Heparanase-driven sequential released nanoparticles for ferroptosis and tumor microenvironment modulations synergism in breast cancer therapy. Biomaterials 2020, 266, 120429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, X.; Li, J.; Luo, Z.; Hu, Y.; Liu, J.; Dai, L.; Zhou, J.; Hou, C.; Cai, K. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology 2015, 26, 145102. [Google Scholar] [CrossRef]
- Liu, K.; Yan, S.; Liu, Z.; Wang, D.; Yang, Q.; Jiang, X.; Chen, L.; Tang, H. New anti-tumor strategy based on acid-triggered self-destructive and near-infrared laser light responses of nano-biocatalysts integrating starvation–chemo–photothermal therapies. Cancer Nanotechnol. 2022, 13, 11. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Qin, X.; Liu, J. Photothermal-Chemotherapy Integrated Nanoparticles with Tumor Microenvironment Response Enhanced the Induction of Immunogenic Cell Death for Colorectal Cancer Efficient Treatment. ACS Appl. Mater. Interfaces 2019, 11, 43393–43408. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, Y.; Lee, A.; Pan, X.; Yang, X.; Zhao, X.; Lee, R.J. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J. Pharm. Pharm. Sci. 2007, 10, 350–357. [Google Scholar]
- Lammers, T.; Subr, V.; Ulbrich, K.; Peschke, P.; Huber, P.E.; Hennink, W.E.; Storm, G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials 2009, 30, 3466–3475. [Google Scholar] [CrossRef]
- Lee, I.-H.; An, S.; Yu, M.K.; Kwon, H.; Im, S.-H.; Jon, S. Targeted chemoimmunotherapy using drug-loaded aptamer–dendrimer bioconjugates. J. Control. Release 2011, 155, 435–441. [Google Scholar] [CrossRef]
- Kaneshiro, T.L.; Lu, Z.-R. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials 2009, 30, 5660–5666. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Yang, L.; Mao, Y.; Wei, Y.; Chen, L. Co-delivery of doxorubicin and plasmid by a novel FGFR-mediated cationic liposome. Int. J. Pharm. 2010, 393, 120–127. [Google Scholar] [CrossRef]
- Zucker, D.; Barenholz, Y. Optimization of vincristine–topotecan combination — Paving the way for improved chemotherapy regimens by nanoliposomes. J. Control. Release 2010, 146, 326–333. [Google Scholar] [CrossRef]
- Song, X.R.; Zheng, Y.; He, G.; Yang, L.; Luo, Y.F.; He, Z.Y.; Li, S.Z.; Li, J.M.; Yu, S.; Luo, X.; et al. Development of PLGA Nanoparticles Simultaneously Loaded with Vincristine and Verapamil for Treatment of Hepatocellular Carcinoma. J. Pharm. Sci. 2010, 99, 4874–4879. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Biganzoli, L.; Coleman, R.; Mauriac, L.; Hennebert, P.; Awada, A.; Nooij, M.; Beex, L.; Piccart, M.; Van Hoorebeeck, I.; et al. EORTC 10968: A phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx®, Doxil®) at a 6-week interval in patients with metastatic breast cancer. Ann. Oncol. 2002, 13, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Silverman, J.A.; Papadopoulos, N.E.; Kim, K.B.; Hagey, A.E.; Vardeleon, A.; Hwu, W.-J.; Homsi, J.; Davies, M.; Hwu, P. Pharmacokinetics and Safety of Marqibo (Vincristine Sulfate Liposomes Injection) in Cancer Patients with Impaired Liver Function. J. Clin. Pharmacol. 2011, 51, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2012, 71, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, D.; Zhang, J.; Jiang, Y.; Song, A.; Li, Z.; Luan, Y. A Three-in-One Immunotherapy Nanoweapon via Cascade-Amplifying Cancer-Immunity Cycle against Tumor Metastasis, Relapse, and Postsurgical Regrowth. Nano Lett. 2019, 19, 6647–6657. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-H.; Kopeček, J. Enhancing Accumulation and Penetration of HPMA Copolymer–Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. J. Am. Chem. Soc. 2015, 137, 6726–6729. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.; Qiu, W.-X.; Hu, J.-J.; Cao, P.-X.; Zhu, C.-H.; Cheng, H.; Zhang, X.-Z. Multifunctional Mesoporous Silica Nanoparticles with Thermal-Responsive Gatekeeper for NIR Light-Triggered Chemo/Photothermal-Therapy. Small 2016, 12, 4286–4298. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-Mediated Release of Chemotherapeutics from Mesoporous Silica Nanoparticles to ex Vivo Human and Mouse Lung Tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef]
- Barnestein, R.; Galland, L.; Kalfeist, L.; Ghiringhelli, F.; Ladoire, S.; Limagne, E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology 2022, 11, 2120676. [Google Scholar] [CrossRef]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2011, 59, 1169–1180. [Google Scholar] [CrossRef]
- Goldie, J.H. Drug Resistance in Cancer: A Perspective. Cancer Metastasis Rev. 2001, 20, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ruan, Y.; Chen, Q.; Yan, S.; Huang, W. Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chem. Eng. J. 2022, 452, 138422. [Google Scholar] [CrossRef]
- Iorio, M.; Ganesh, N.U.; De Luise, M.; Porcelli, A.M.; Gasparre, G.; Kurelac, I. The Neglected Liaison: Targeting Cancer Cell Metabolic Reprogramming Modifies the Composition of Non-Malignant Populations of the Tumor Microenvironment. Cancers 2021, 13, 5447. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, F.; Liu, L.; Li, L.; Huang, J.; Wang, L.; Tang, Y.; Ke, B.; Song, L.; Cheng, C.; Ma, L.; et al. Conjugated Coordination Porphyrin-based Nanozymes for Photo-/Sono-Augmented Biocatalytic and Homologous Tumor Treatments. ACS Appl. Mater. Interfaces 2021, 13, 41485–41497. [Google Scholar] [CrossRef]
- Yang, J.; Yao, H.; Guo, Y.; Yang, B.; Shi, J. Enhancing Tumor Catalytic Therapy by Co-Catalysis. Angew. Chem. 2022, 134, e202200480. [Google Scholar] [CrossRef]
- Pavel, M.; Gradinariu, G.; Stancu, A. Study of the Optimum Dose of Ferromagnetic Nanoparticles Suitable for Cancer Therapy Using MFH. IEEE Trans. Magn. 2008, 44, 3205–3208. [Google Scholar] [CrossRef]
- Pavel, M.; Stancu, A. Ferromagnetic Nanoparticles Dose Based on Tumor Size in Magnetic Fluid Hyperthermia Cancer Therapy. IEEE Trans. Magn. 2009, 45, 5251–5254. [Google Scholar] [CrossRef]
- Hou, H.; Huang, X.; Wei, G.; Xu, F.; Wang, Y.; Zhou, S. Fenton Reaction-Assisted Photodynamic Therapy for Cancer with Multifunctional Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 29579–29592. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, T.; Li, Y.; Lau, J.; Yang, Z.; Fan, W.; Zhou, Z.; Shi, C.; Ke, C.; Bregadze, V.I.; et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 2018, 12, 11355–11365. [Google Scholar] [CrossRef]
- Chi, H.; Zhu, G.; Yin, Y.; Diao, H.; Liu, Z.; Sun, S.; Guo, Z.; Xu, W.; Xu, J.; Cui, C.; et al. Dual-Responsive multifunctional “core–shell” magnetic nanoparticles promoting Fenton reaction for tumor ferroptosis therapy. Int. J. Pharm. 2022, 622, 121898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, X.; Zou, M.; Ding, B.; Xiao, H.; Wang, M.; Jiang, F.; Cheng, Z.; Ma, P.; Lin, J. Retracted: Nanozyme-Initiated In Situ Cascade Reactions for Self-Amplified Biocatalytic Immunotherapy. Adv. Mater. 2020, 33, e2006363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ji, T.; Wang, H.; Li, S.; Zhao, Y.; Nie, G. Self-assembled peptide nanoparticles as tumor microenvironment activatable probes for tumor targeting and imaging. J. Control. Release 2014, 177, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Jin, W.; Zhang, T.; Zhang, F.; Gong, L.; Liu, X.; Deng, X.; Gao, W. Self-Activated Cascade Biocatalysis of Glucose Oxidase–Polycation–Iron Nanoconjugates Augments Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 32823–32835. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, 2104223. [Google Scholar] [CrossRef]
- Shao, L.; Gao, X.; Liu, J.; Zheng, Q.; Li, Y.; Yu, P.; Wang, M.; Mao, L. Biodegradable Metal–Organic-Frameworks-Mediated Protein Delivery Enables Intracellular Cascade Biocatalysis and Pyroptosis In Vivo. ACS Appl. Mater. Interfaces 2022, 14, 47472–47481. [Google Scholar] [CrossRef]
- Wang, R.; Yan, C.; Zhang, H.; Guo, Z.; Zhu, W.-H. In vivo real-time tracking of tumor-specific biocatalysis in cascade nanotheranostics enables synergistic cancer treatment. Chem. Sci. 2020, 11, 3371–3377. [Google Scholar] [CrossRef] [Green Version]
- Presnell, C.E.; Bhatti, G.; Numan, L.; Lerche, M.; Alkhateeb, K.S.; Ghalib, M.; Shammaa, M.; Kavdia, M. Computational insights into the role of glutathione in oxidative stress. Curr. Neurovascular Res. 2013, 10, 185–194. [Google Scholar] [CrossRef]
- Secret, E.; Kelly, S.J.; Crannell, K.E.; Andrew, J.S. Enzyme-Responsive Hydrogel Microparticles for Pulmonary Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 10313–10321. [Google Scholar] [CrossRef]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Liu, Z.; Wu, H.; An, P.; Zhi, X.; Yin, Y.; Liu, W.; Sun, B. Dual catalytic cascaded nanoplatform for photo/chemodynamic/starvation synergistic therapy. Colloids Surfaces B Biointerfaces 2020, 199, 111538. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, L.; Liang, K.; Cheong, S.; Boyer, C.; Gooding, J.J.; Chen, Y.; Gu, Z. Biodegradable 2D Fe-Al Hydroxide for Nanocatalytic Tumor-Dynamic Therapy with Tumor Specificity. Adv. Sci. 2018, 5, 1801155. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Ma, Y.; Liu, Y.; Yan, C.; Shi, P.; Tian, H.; Zhu, W.-H. Photocaged prodrug under NIR light-triggering with dual-channel fluorescence: In vivo real-time tracking for precise drug delivery. Sci. China Chem. 2018, 61, 1293–1300. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Pandey, S.; Kumar, D. Importance of photophosphatidylserine and Tim-3 in photoimmunotherapy. RSC Med. Chem. 2022, 13, 1274–1275. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilfi, A.; Walker, K.D. Biocatalysis of precursors to new-generation SB-T-taxanes effective against paclitaxel-resistant cancer cells. Arch. Biochem. Biophys. 2022, 719, 109165. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Z.; Zhang, Q.; Xu, J.; Yun, S.L.J.; Liang, K.; Gu, Z. Chemotaxis-Driven 2D Nanosheet for Directional Drug Delivery toward the Tumor Microenvironment. Small 2020, 16, e2002732. [Google Scholar] [CrossRef] [PubMed]
- Philpott, H.K.; Thomas, P.J.; Tew, D.; Fuerst, D.E.; Lovelock, S.L. A versatile biosynthetic approach to amide bond formation. Green Chem. 2018, 20, 3426–3431. [Google Scholar] [CrossRef]
- Nishimura, Y. Losmapimod: A Novel Clinical Drug to Overcome Gefitinib-Resistance. Ebiomedicine 2018, 28, 2–3. [Google Scholar] [CrossRef]
- Petchey, M.; Cuetos, A.; Rowlinson, B.; Dannevald, S.; Frese, A.; Sutton, P.W.; Lovelock, S.; Lloyd, R.C.; Fairlamb, I.J.S.; Grogan, G. The Broad Aryl Acid Specificity of the Amide Bond Synthetase McbA Suggests Potential for the Biocatalytic Synthesis of Amides. Angew. Chem. Int. Ed. 2018, 57, 11584–11588. [Google Scholar] [CrossRef] [Green Version]
- Petchey, M.R.; Rowlinson, B.; Lloyd, R.C.; Fairlamb, I.J.S.; Grogan, G. Biocatalytic Synthesis of Moclobemide Using the Amide Bond Synthetase McbA Coupled with an ATP Recycling System. ACS Catal. 2020, 10, 4659–4663. [Google Scholar] [CrossRef]
- Meenu, M.; Verma, V.K.; Seth, A.; Sahoo, R.K.; Gupta, P.; Arya, D.S. Association of Monoamine Oxidase A with Tumor Burden and Castration Resistance in Prostate Cancer. Curr. Ther. Res. 2020, 93, 100610. [Google Scholar] [CrossRef] [PubMed]
- Lubberink, M.; Schnepel, C.; Citoler, J.; Derrington, S.R.; Finnigan, W.; Hayes, M.A.; Turner, N.J.; Flitsch, S.L. Biocatalytic Monoacylation of Symmetrical Diamines and Its Application to the Synthesis of Pharmaceutically Relevant Amides. ACS Catal. 2020, 10, 10005–10009. [Google Scholar] [CrossRef]
- Wood, A.; Weise, N.J.; Frampton, J.D.; Dunstan, M.S.; Hollas, M.A.; Derrington, S.R.; Lloyd, R.C.; Quaglia, D.; Parmeggiani, F.; Leys, D.; et al. Adenylation Activity of Carboxylic Acid Reductases Enables the Synthesis of Amides. Angew. Chem. 2017, 129, 14690–14693. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Qu, Z.; Qi, Y.; Zhan, S. Current situation and challenge of registry in China. Front. Med. 2014, 8, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Saaidi, P.-L.; Monfleur, A.; Harari, M.; Cuccaro, J.; Fossey, A.; Besnard, M.; Debard, A.; Mariage, A.; Pellouin, V.; et al. Synthesis of Mono- and Dihydroxylated Amino Acids with New α-Ketoglutarate-Dependent Dioxygenases: Biocatalytic Oxidation of C-H Bonds. Chemcatchem 2014, 6, 3012–3017. [Google Scholar] [CrossRef] [Green Version]
- Hara, R.; Yamagata, K.; Miyake, R.; Kawabata, H.; Uehara, H.; Kino, K. Discovery of Lysine Hydroxylases in the Clavaminic Acid Synthase-Like Superfamily for Efficient Hydroxylysine Bioproduction. Appl. Environ. Microbiol. 2017, 83, e00693-17. [Google Scholar] [CrossRef] [Green Version]
- Amatuni, A.; Renata, H. Identification of a lysine 4-hydroxylase from the glidobactin biosynthesis and evaluation of its biocatalytic potential. Org. Biomol. Chem. 2018, 17, 1736–1739. [Google Scholar] [CrossRef]
- Zhang, X.; King-Smith, E.; Renata, H. Total Synthesis of Tambromycin by Combining Chemocatalytic and Biocatalytic C−H Functionalization. Angew. Chem. Int. Ed. 2018, 57, 5037–5041. [Google Scholar] [CrossRef]
- Amatuni, A.; Shuster, A.; Adibekian, A.; Renata, H. Concise Chemoenzymatic Total Synthesis and Identification of Cellular Targets of Cepafungin I. Cell Chem. Biol. 2020, 27, 1318–1326.e18. [Google Scholar] [CrossRef]
- Schober, M.; MacDermaid, C.; Ollis, A.A.; Chang, S.; Khan, D.; Hosford, J.; Latham, J.; Ihnken, L.A.F.; Brown, M.J.B.; Fuerst, D.; et al. Chiral synthesis of LSD1 inhibitor GSK2879552 enabled by directed evolution of an imine reductase. Nat. Catal. 2019, 2, 909–915. [Google Scholar] [CrossRef]
- Bauer, T.M.; Besse, B.; Martinez-Marti, A.; Trigo, J.M.; Moreno, V.; Garrido, P.; Ferron-Brady, G.; Wu, Y.; Park, J.; Collingwood, T.; et al. Phase I, Open-Label, Dose-Escalation Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Efficacy of GSK2879552 in Relapsed/Refractory SCLC. J. Thorac. Oncol. 2019, 14, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; Yee, K.; Verma, A.; Borthakur, G.; Burguera, A.D.L.F.; Sanz, G.; Mohammad, H.P.; Kruger, R.G.; Karpinich, N.O.; Ferron-Brady, G.; et al. Phase I trials of the lysine-specific demethylase 1 inhibitor, GSK2879552, as mono- and combination-therapy in relapsed/refractory acute myeloid leukemia or high-risk myelodysplastic syndromes. Leuk. Lymphoma 2021, 63, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.-H.; Memmert, K. Epothilones as lead structures for new anticancer drugs—Pharmacology, fermentation, and structure-activity-relationships. Nat. Compd. Drugs 2008, 66, 273–334. [Google Scholar] [CrossRef]
- Nayeem, A.; Chiang, S.-J.; Liu, S.-W.; Sun, Y.; You, L.; Basch, J. Engineering enzymes for improved catalytic efficiency: A computational study of site mutagenesis in epothilone-B hydroxylase. Protein Eng. Des. Sel. 2009, 22, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, M.L.; Watts, R.E.; Khosla, C. Substrate Tolerance of Module 6 of the Epothilone Synthetase. Biochemistry 2007, 46, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N. Biocatalysis: Synthesis of Key Intermediates for Development of Pharmaceuticals. ACS Catal. 2011, 1, 1056–1074. [Google Scholar] [CrossRef]
- Meyer, F.; Frey, R.; Ligibel, M.; Sager, E.; Schroer, K.; Snajdrova, R.; Buller, R. Modulating Chemoselectivity in a Fe(II)/α-Ketoglutarate-Dependent Dioxygenase for the Oxidative Modification of a Nonproteinogenic Amino Acid. ACS Catal. 2021, 11, 6261–6269. [Google Scholar] [CrossRef]
- Seide, S.; Arnold, L.; Wetzels, S.; Bregu, M.; Gätgens, J.; Pohl, M. From Enzyme to Preparative Cascade Reactions with Immobilized Enzymes: Tuning Fe(II)/α-Ketoglutarate-Dependent Lysine Hydroxylases for Application in Biotransformations. Catalysts 2022, 12, 354. [Google Scholar] [CrossRef]
- Felnagle, E.A.; Jackson, E.E.; Chan, Y.A.; Podevels, A.M.; Berti, A.D.; McMahon, M.D.; Thomas, M.G. Nonribosomal Peptide Synthetases Involved in the Production of Medically Relevant Natural Products. Mol. Pharm. 2008, 5, 191–211. [Google Scholar] [CrossRef]













| Drugs | Drug-Delivery System | Biocatalysts Used | Results (Targeted Site/Nanomedicine Produces) | Ref. |
|---|---|---|---|---|
| Doxorubicin | Mesoporous silica nanoparticle | MMP-13 | Reduced side effects as a targeting moiety and end-capping agent. | [54] |
| Doxorubicin + verapamil | Transferrin-conjugated PEGylated liposome | cytochrome P450 oxidase | To treat leukemia, efficacy for liposomal loading was observed to be 95% and 70% of DOX and VER, respectively. | [57] |
| Gemcitabine + doxorubicin | HPMA-Gem-Dox | MMP-2 | In prostate cancer, it was observed that polymers in the form of liposomes could be utilized to deliver multiple chemotherapeutic drugs at the in vivo tumor sites simultaneously. | [58] |
| Unmethylated CpG-ONTs + doxorubicin | Aptamer-G4 PAMAM dendrimer conjugates | cytochrome P450 oxidase | Chemo-immunotherapy system to treat prostate cancer. | [59] |
| Doxorubicin + siRNA | RGDfK-G3 poly-lysine dendrimer | cytochrome P450 oxidase | Compared to free doxorubicin at high doses, the nanoparticle formed showed higher cytotoxicity in glioblastoma U87 cells. | [60] |
| Doxorubicin + Msurvivin T34A plasmid | Liposome | cytochrome P450 oxidase | Inhibit tumor growth in the Lewis lung-carcinoma-bearing C57BL/6 mice. | [61] |
| Topotecan + vincristine | PEG-liposome | Strictosidine synthase (STR) to produce the intermediate Strictosidine, and peroxidases for the production of Vincristine. | Delivery of the drugs simultaneously at a defined ratio to the cancer site showed more efficiency. | [62] |
| Vincristine + verapamil | PLGA | Strictosidine synthase (STR) to produce the intermediate Strictosidine and peroxidases for the production of Vincristine. | Treatment of drug-resistant human hepatocellular carcinoma in vivo. | [63] |
| Doxorubicin | Liposome | cytochrome P450 oxidase | Doxil® (US FDA approved nanomedicines) | [64] |
| Vincristine | Liposome | Strictosidine synthase (STR) to produce the intermediate Strictosidine, and peroxidases for the production of Vincristine. | Marqibo (US FDA approved nanomedicines) | [65,66] |
| Indoximod (NLG-8189) IDO1 inhibitors | Ce6-conjugated hyaluronic acid, Indoximod-conjugated polylysine, and aPD-L1. | 2,3-dioxygenase (IDO) pathway inhibitorS 1-mt | Increase the effectiveness of immunotherapy, prevent tumor metastasis, and postoperative regeneration and regrowth. | [67] |
| Doxorubicin | HPMA copolymer | MMP-2 | Increased entry of DOX in DU-145 cells in the presence of MMP-2 observed in prostate cancer cells. | [68] |
| Doxorubicin | Mesoporous silica nanoparticle | MMP-2 | The photothermal molecules indocyanine green (ICG) and DOX were detected using PEG-MSN. | [69] |
| Cisplatin | Mesoporous silica nanoparticle | MMP-9 | Cleave heptapeptide connected to a biotin group, which is coated on the outside of MSNs, as a result of the heptapeptide sequence’s selective proteolysis. | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, S.; Das, P.; Sharma, S.; Shukla, M.K.; Kumar, R.; Kumar Tonk, R.; Pandey, S.; Kumar, D. Role and Application of Biocatalysts in Cancer Drug Discovery. Catalysts 2023, 13, 250. https://doi.org/10.3390/catal13020250
Sengupta S, Das P, Sharma S, Shukla MK, Kumar R, Kumar Tonk R, Pandey S, Kumar D. Role and Application of Biocatalysts in Cancer Drug Discovery. Catalysts. 2023; 13(2):250. https://doi.org/10.3390/catal13020250
Chicago/Turabian StyleSengupta, Sounok, Prathama Das, Samridhi Sharma, Monu Kumar Shukla, Rajesh Kumar, Rajiv Kumar Tonk, Sadanand Pandey, and Deepak Kumar. 2023. "Role and Application of Biocatalysts in Cancer Drug Discovery" Catalysts 13, no. 2: 250. https://doi.org/10.3390/catal13020250
APA StyleSengupta, S., Das, P., Sharma, S., Shukla, M. K., Kumar, R., Kumar Tonk, R., Pandey, S., & Kumar, D. (2023). Role and Application of Biocatalysts in Cancer Drug Discovery. Catalysts, 13(2), 250. https://doi.org/10.3390/catal13020250