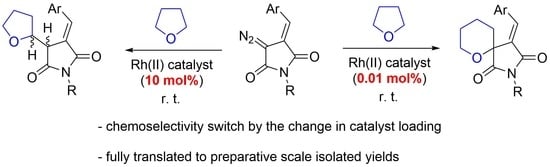
Catalyst Loading Controls Chemoselectivity: Unusual Effect in Rhodium(II) Carbene Insertion Reactions with Tetrahydrofuran †
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
Performance of 1a → 2a + 3a Reaction on Analytical Scale
3.2. Reactions of DAS Compounds 1a–d with THF under Rh(II) Catalysis (Preparative Experiments)
3.2.1. (E)-2-Benzyl-4-benzylidene-6-oxa-2-azaspiro [4.5]decane-1,3-dione (2a)
3.2.2. (E)-1-Benzyl-3-benzylidene-4-(tetrahydrofuran-2-yl)pyrrolidine-2,5-dione (3a)
3.2.3. (E)-4-Benzylidene-2-phenyl-6-oxa-2-azaspiro [4.5]decane-1,3-dione (2b)
3.2.4. (E)-3-Benzylidene-1-phenyl-4-(tetrahydrofuran-2-yl)pyrrolidine-2,5-dione (3b)
3.2.5. (E)-4-Benzylidene-2-(4-fluorophenyl)-6-oxa-2-azaspiro [4.5]decane-1,3-dione (2c)
3.2.6. (E)-3-Benzylidene-1-(4-fluorophenyl)-4-(tetrahydrofuran-2-yl)pyrrolidine-2,5-dione (3c)
3.2.7. (E)-4-(4-Fluorobenzylidene)-2-phenyl-6-oxa-2-azaspiro [4.5]decane-1,3-dione (2d)
3.2.8. (E)-3-(4-Fluorobenzylidene)-1-phenyl-4-(tetrahydrofuran-2-yl)pyrrolidine-2,5-dione (3d)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, T.; McKervey, M.A. Organic Synthesis with alpha-Diazo Carbonyl Compounds. Chem. Rev. 1994, 94, 1091–1160. [Google Scholar]
- Chupakhin, E.; Gecht, M.; Ivanov, A.; Kantin, G.; Dar’in, D.; Krasavin, M. (E)-3-Arylidene-4-diazopyrrolidine-2,5-diones: Preparation and use in RhII-catalyzed X–H insertion reactions towards novel, medicinally important Michael acceptors. Synthesis 2021, 53, 1292–1300. [Google Scholar]
- Doyle, M.P.; Duffy, R.; Ratnikov, M.; Zhou, L. Catalytic Carbene Insertion into C−H Bonds. Chem. Rev. 2010, 110, 704–724. [Google Scholar]
- Guranova, N.I.; Dar’in, D.; Kantin, G.; Novikov, A.S.; Bakulina, O.; Krasavin, M. Rh(II)-Catalyzed Spirocyclization of α-Diazo Homophthalimides with Cyclic Ethers. J. Org. Chem. 2019, 84, 4534–4542. [Google Scholar]
- Wang, H.; Guptill, D.M.; Alvarez, A.V.; Musaev, D.G.; Davies, H.M.L. Rhodium-catalyzed enantioselective cyclopropanation of electron deficient alkenes. Chem. Sci. 2013, 4, 2844–2850. [Google Scholar]
- Laha, D.; Bhat, R.G. Silver-Catalyzed Epoxidation of Aldehydes Using Donor-/Acceptor-type Vinyl Diazosuccinimides to Access Spiro Pyrrolidinedione Oxiranes. Asian J. Org. Chem. 2020, 9, 918–921. [Google Scholar]
- Budeev, A.; Kantin, G.; Dar’in, D.; Krasavin, M. Diazocarbonyl and Related Compounds in the Synthesis of Azoles. Molecules 2021, 26, 2530. [Google Scholar]
- Dar’in, D.; Kantin, G.; Bakulina, O.; Inyutina, A.; Chupakhin, E.; Krasavin, M. Spirocyclizations involving oxonium ylides derived from cyclic α-diazocarbonyl compounds: An entry into 6-oxa-2-azaspiro [4.5]decane scaffold. J. Org. Chem. 2020, 85, 15586–15599. [Google Scholar]
- Inyutina, A.; Dar’in, D.; Kantin, G.; Krasavin, M. Tricyclic 2-Benzazepines Obtained via an Unexpected Cyclization Involving Nitrilium Ylides. Org. Biomol. Chem. 2021, 19, 5068–5071. [Google Scholar]
- Inyutina, A.; Kantin, G.; Dar’in, D.; Krasavin, M. Diastereoselective Formal [5+2] Cycloaddition of Diazo Arylidene Succinimides-Derived Rhodium Carbenes and Aldehydes: A Route to 2-Benzoxepines. J. Org. Chem. 2021, 86, 13673–13683. [Google Scholar]
- Vepreva, A.; Kantin, G.; Krasavin, M.; Dar’in, D. A General Way to Spiro-Annulated 2-Benzoxepines via Rh2(esp)2-catalyzed [5+2] Cycloaddition of Diazo Arylidene Succinimides to Ketones. Synthesis 2022, 54, 5128–5138. [Google Scholar]
- Vepreva, A.; Bunev, A.S.; Kudinov, A.Y.; Kantin, G.; Krasavin, M.; Dar’in, D. Unusual Highly Diastereoselective Rh(II)-catalyzed Dimerization of Diazo Arylidene Succinimides Provides Access to New Dibenzazulene Scaffold. Beilstein J. Org. Chem. 2022, 18, 533–538. [Google Scholar]
- Nakamura, E.; Yoshikai, N.; Yamanaka, M. Mechanism of C−H Bond Activation/C−C Bond Formation Reaction between Diazo Compound and Alkane Catalyzed by Dirhodium Tetracarboxylate. J. Am. Chem. Soc. 2002, 124, 7181–7192. [Google Scholar]
- Chupakhin, E.G.; Kantin, G.P.; Dar’in, D.V.; Krasavin, M. Convenient preparation of (E)-3-arylidene-4-diazopyrrolidine-2,5-diones in array format. Mendeleev Commun. 2021, 31, 36–38. [Google Scholar]








 | ||||
|---|---|---|---|---|
| Time, min | Conversion (%) | Yield of 2a (%) | Yield of 3a (%) | Ratio 2a:3a |
| 2 | 2 | 2 | 1 | 69:31 |
| 15 | 16 | 10 | 5 | 67:33 |
| 30 | 29 | 23 | 11 | 68:32 |
| 45 | 41 | 27 | 13 | 67:33 |
| 60 | 51 | 34 | 16 | 66:34 |
| 90 | 67 | 39 | 21 | 65:35 |
| 120 | 76 | 47 | 24 | 66:34 |
| 150 | 84 | 50 | 25 | 65:35 |
| 180 | 89 | 52 | 27 | 66:34 |
| 240 | 95 | 55 | 29 | 65:35 |
| 300 | 98 | 57 | 30 | 65:35 |
| 360 | 100 | 59 | 32 | 64:36 |
| THF V (mL) | C (1a), mg/mL | Yield of 2a (%) | Yield of 3a (%) | Fraction of 3a (%) |
|---|---|---|---|---|
| 0.5 | 60.6 | 28 | 72 | 72 |
| 1.0 | 30.3 | 38 | 62 | 62 |
| 3.0 | 15.2 | 47 | 41 | 47 |
| 5.0 | 7.6 | 41 | 25 | 38 |
| 10.0 | 3.8 | 56 | 29 | 34 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Substrate | Ar | R | Catalyst | mol% | Yield 2 (%) | Yield 3 (%) |
| 1 | 1a | Ph | Bn | Rh2(OAc)4 | 10 | 15 | 38 |
| 2 | 1a | Ph | Bn | Rh2(OAc)4 | 0.01 | 46 | 7 |
| 3 | 1a | Ph | Bn | Rh2(AdmO)4 | 10 | 10 | 72 |
| 4 | 1a | Ph | Bn | Rh2(AdmO)4 | 0.01 | 49 | 30 |
| 5 | 1b | Ph | Ph | Rh2(OAc)4 | 10 | 26 | 60 |
| 6 | 1b | Ph | Ph | Rh2(OAc)4 | 0.01 | 78 | 20 |
| 7 | 1b | Ph | Ph | Rh2(AdmO)4 | 10 | 7 | 76 |
| 8 | 1b | Ph | Ph | Rh2(AdmO)4 | 0.01 | 75 | 9 |
| 9 | 1c | Ph | 4-FC6H4 | Rh2(OAc)4 | 10 | 14 | 43 |
| 10 | 1c | Ph | 4-FC6H4 | Rh2(OAc)4 | 0.01 | 72 | 4 |
| 11 | 1d | 4-FC6H4 | Ph | Rh2(OAc)4 | 10 | 20 | 66 |
| 12 | 1d | 4-FC6H4 | Ph | Rh2(OAc)4 | 0.01 | 69 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazantsev, A.; Rodionov, I.A.; Bakulina, O.; Kantin, G.; Dar’in, D.; Krasavin, M. Catalyst Loading Controls Chemoselectivity: Unusual Effect in Rhodium(II) Carbene Insertion Reactions with Tetrahydrofuran. Catalysts 2023, 13, 428. https://doi.org/10.3390/catal13020428
Kazantsev A, Rodionov IA, Bakulina O, Kantin G, Dar’in D, Krasavin M. Catalyst Loading Controls Chemoselectivity: Unusual Effect in Rhodium(II) Carbene Insertion Reactions with Tetrahydrofuran. Catalysts. 2023; 13(2):428. https://doi.org/10.3390/catal13020428
Chicago/Turabian StyleKazantsev, Alexander, Ivan A. Rodionov, Olga Bakulina, Grigory Kantin, Dmitry Dar’in, and Mikhail Krasavin. 2023. "Catalyst Loading Controls Chemoselectivity: Unusual Effect in Rhodium(II) Carbene Insertion Reactions with Tetrahydrofuran" Catalysts 13, no. 2: 428. https://doi.org/10.3390/catal13020428
APA StyleKazantsev, A., Rodionov, I. A., Bakulina, O., Kantin, G., Dar’in, D., & Krasavin, M. (2023). Catalyst Loading Controls Chemoselectivity: Unusual Effect in Rhodium(II) Carbene Insertion Reactions with Tetrahydrofuran. Catalysts, 13(2), 428. https://doi.org/10.3390/catal13020428