Photocatalytic CO2 Reduction to CH4 and Dye Degradation Using Bismuth Oxychloride/Bismuth Oxyiodide/Graphitic Carbon Nitride (BiOmCln/BiOpIq/g-C3N4) Nanocomposite with Enhanced Visible-Light Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Produced BiOmCln/BiOpIq/g-C3N4 Composites
2.1.1. XRD Analysis
2.1.2. SEM and TEM
2.1.3. Analysis of XPS Spectra
2.1.4. UV–Vis DRS Analysis
2.1.5. PL Analysis
2.1.6. BET Analysis
2.2. Photocatalytic Activity
2.2.1. Photocatalytic Reduction of CO2
2.2.2. Photocatalytic Degradation of CV
2.2.3. Reuse of BiOCl/BiOI/g-C3N4
2.2.4. Verification of Active Species and EPR Analysis
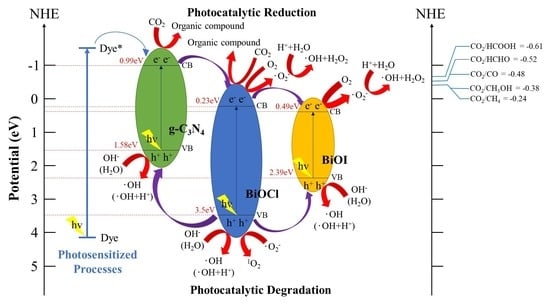
2.3. Schematic of Bandgap Structures of BiOCl/BiOI/g-C3N4
3. Experimental
3.1. Materials
3.2. Instruments and Analytical Methods
3.3. Synthesis of Different BiOmCln/BiOpIq/g-C3N4 Composites
3.4. Photocatalytic Experiments
3.4.1. Reduction of CO2
3.4.2. Degradation of Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.H.; Porta, M.; Jacobs, D.R.; Vandenberg, L.N., Jr. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 2014, 35, 557–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.L.; Liu, F.Y.; Xiao, X.; Hu, J.; Gao, B.; Zou, D.; Chen, C.C. Visible-light-driven photocatalysis of carbon dioxide and organic pollutants by MFeO2 (M = Li, Na, or K). J. Colloid Interface Sci. 2021, 601, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; Dai, Y.M.; Chen, F.H.; Chen, C.C. Lead bismuth oxybromide/graphene oxide: Synthesis, characterization, and photocatalytic activity for removal of carbon dioxide, crystal violet dye, and 2-hydroxybenzoic acid. J. Colloid Interface Sci. 2020, 562, 112–124. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, J.; Hou, Y.; Wang, Y.; Yang, S.; Yang, H.G. Surface chelation of cesium halide perovskite by dithiocarbamate for efficient and stable solar cells. Nat. Commun. 2020, 11, 4237. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Velilla, E.; Montoya, J.F.; Jaramillo, F. Mitigating scalability issues of perovskite photovoltaic technology through a p-i-n meso-superstructured solar cell architecture. Sol. Energy Mater. Sol. Cells 2019, 195, 191–197. [Google Scholar] [CrossRef]
- Zheng, J.; Lei, Z. Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl. Catal. B Environ. 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Yin, R.; Li, Y.; Zhong, K.; Yao, H.; Zhang, Y.; Lai, K. Multifunctional property exploration: Bi4O5I2 with high visible light photocatalytic performance and a large nonlinear optical effect. RSC Adv. 2019, 9, 4539–4544. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Zhu, L.; Wang, S.; Chu, X.; Yue, L. Novel mesoporous graphite carbon nitride/BiOI heterojunction for enhancing photocatalytic performance under visible-light irradiation. Appl. Mater. Interfaces 2014, 6, 5083–5093. [Google Scholar] [CrossRef]
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, P.Q.; Liu, J.Y.; Liu, X.J. Enhanced photocatalytic performance of direct Z-scheme BiOCl–g-C3N4 photocatalysts. RSC Adv. 2014, 4, 19456. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, J.; Li, Y.; Liu, Q.; Zhang, X. Enhanced photocatalytic activity of BiOI under visible light irradiation by the modification of MoS2. RSC Adv. 2017, 7, 42398–42406. [Google Scholar] [CrossRef] [Green Version]
- Bhachu, D.S.; Moniz, S.J.A.; Sathasivam, S.; Scanlon, D.O.; Walsh, A.; Bawaked, S.M.; Mokhtar, M.; Obaid, A.Y.; Parkin, I.P.; Tang, J.; et al. Bismuth oxyhalides: Synthesis, structure and photoelectrochemical activity. Chem. Sci. 2016, 7, 4832–4841. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kumar, A.; Krishnan, V. Influence of different bismuth oxyhalides on the photocatalytic activity of graphitic carbon nitride: A comparative study under natural sunlight. Mater. Adv. 2020, 1, 1262–1272. [Google Scholar] [CrossRef]
- Zheng, M.; Ma, X.; Hu, J.; Zhang, X.; Duan, W. Novel recyclable BiOBr/Fe3O4/RGO composites with remarkable visible-light photocatalytic activity. RSC Adv. 2020, 10, 19961–19973. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, L.; Zhu, J.; Chen, M.; Shi, W.; Xie, J. Novel p-n heterojunction photocatalyst constructed by porous graphite-like C3N4 and nanostructured BiOI: Facile synthesis and enhanced photocatalytic activity. Dalton Trans. 2013, 42, 15726–15734. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nano composites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Yang, C.T.; Lee, W.W.; Lin, H.P.; Dai, Y.M.; Chi, H.T.; Chen, C.C. A novel heterojunction photocatalyst, Bi2SiO5/g-C3N4 synthesis, characterization, photocatalytic activity, and mechanism. RSC Adv. 2016, 6, 40664. [Google Scholar] [CrossRef]
- Lin, H.P.; Chen, C.C.; Lee, W.W.; Lai, Y.Y.; Chen, J.Y.; Chen, Y.Q.; Fu, J.Y. Synthesis of a SrFeO3-x/g-C3N4 heterojunction with improved visible-light photocatalytic activities in chloramphenicol and crystal violet degradation. RSC Adv. 2016, 6, 2323. [Google Scholar] [CrossRef]
- Chou, S.Y.; Chen, C.C.; Dai, Y.M.; Lin, J.H.; Lee, W.W. Novel synthesis of bismuth oxyiodidegraphitic carbon nitride nanocomposites with enhanced visible-light photocatalytic activity. RSC Adv. 2016, 6, 33478–33491. [Google Scholar] [CrossRef]
- Wang, B.; Di, J.; Liu, G.; Yin, S.; Xia, J.; Zhang, Q.; Li, H. Novel mesoporous graphitic carbon nitride modified PbBiO2Br porous microspheres with enhanced photocatalytic performance. J. Colloid Interface Sci. 2017, 507, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Wang, Y.C.; Chen, C.C. Composite photocatalyst, tetragonal lead bismuth oxyiodide/bismuth oxyiodide/graphitic carbon nitride synthesis, characterization, and photocatalytic activity. J. Colloid Interface Sci. 2019, 533, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lee, A.H.; Chen, C.C. Perovskite-like photocatalyst, PbBiO2/BrPbO/g-C3N4 synthesis, characterization, and visible-light-driven photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2018, 93, 315–328. [Google Scholar] [CrossRef]
- Li, Z.; Feng, J.; Yan, S.; Zou, Z. Solar fuel production: Strategies and new opportunities with nanostructures. Nano Today 2015, 10, 468–486. [Google Scholar] [CrossRef]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-driven metal halide perovskite photocatalysis: Design, stability, and performance. Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Cheng, M.; Lei, L.; Chen, Y.; Zhou, C.; Deng, R.; Li, B. Surface and interface engineering of two-dimensional bismuth-based photocatalysts for ambient molecule activation. J. Mater. Chem. A 2021, 9, 196–233. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Chen, H.; Weng, Y.X.; Tang, H.; Chen, Z.; Zhu, W.; She, Y.; Xia, J.; Li, H. Unique Z-scheme carbonized polymer dots/Bi4O5Br2 hybrids for efficiently boosting photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 293, 120128. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Wang, J.; Wu, X.; Zhang, G. Sb2WO6/BiOBr 2D nanocomposite S-scheme photocatalyst for NO removal. J. Mater. Sci. Technol. 2020, 56, 236–243. [Google Scholar] [CrossRef]
- Chen, C.C.; Fu, J.Y.; Chang, J.L.; Huang, S.T.; Yeh, T.W.; Huang, J.T.; Huang, P.H.; Liu, F.Y.; Chen, L.W. Bismuth oxyfluoride/bismuth oxyiodide nanocomposites enhance visible-light-driven photocatalytic activity. J. Colloid Interface Sci. 2018, 532, 375–386. [Google Scholar] [CrossRef]
- Chou, Y.C.; Lin, Y.Y.; Lu, C.S.; Liu, F.Y.; Lin, J.H.; Chen, F.H.; Chen, C.C.; Wu, W.T. Controlled hydrothermal synthesis of BiOxCly/BiOmBrn/g-C3N4 composites exhibiting visible-light photocatalytic activity. J. Environ. Manag. 2021, 297, 113256. [Google Scholar] [CrossRef]
- Siao, C.W.; Lee, W.L.W.; Dai, Y.M.; Chung, W.H.; Hung, J.T.; Hung, P.H.; Lin, W.Y.; Chen, C.C. BiOxCly/BiOmBrn/BiOpIq/GO quaternary composites syntheses and application of visible-light-driven photocatalytic activities. J. Colloid Interface Sci. 2019, 544, 25–36. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, Q.; Li, F.T.; Yang, W.Y.; Zhao, Y.; Hao, Y.J.; Liu, S.J. Novel BiOCl–C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J. 2013, 234, 361–371. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2013, 142–143, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.R.; Lin, H.P.; Chung, W.H.; Dai, Y.M.; Lin, W.Y.; Chen, C.C. Controlled hydrothermal synthesis of BiOxCly/BiOmIn composites exhibiting visible-light photocatalytic degradation of crystal violet. J. Hazard. Mater. 2015, 283, 787–805. [Google Scholar] [CrossRef]
- Lee, W.W.; Lu, C.S.; Chuang, C.W.; Chen, Y.J.; Fu, J.Y.; Siao, C.W.; Chen, C.C. Synthesis of bismuth oxyiodides and their composites: Characterization, photocatalytic activity, and degradation mechanisms. RSC Adv. 2015, 5, 23450–23463. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, C.; Hu, R.; Zuo, X.; Nan, J.; Li, L.; Wang, L. Oxygen-rich bismuth oxyhalides: Generalized one-pot synthesis, band structures and visible-light photocatalytic properties. J. Mater. Chem. 2012, 22, 22840–22843. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, T.T.; Shaya, J.; Wu, C.L.; Lu, C.S. Bi12SiO20/g-C3N4 heterojunctions: Synthesis, characterization, photocatalytic activity for organic pollutant degradation, and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 123, 228–244. [Google Scholar] [CrossRef]
- Li, T.B.; Chen, G.; Zhou, C.; Shen, Z.Y.; Jin, R.C.; Sun, J.X. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances. Dalton Trans. 2011, 40, 6751–6758. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Wei, Q. Porous BiOI sonocatalysts: Hydrothermal synthesis, characterization, sonocatalytic, and kinetic properties. Ind. Eng. Chem. Res. 2012, 51, 1193–1197. [Google Scholar] [CrossRef]
- Zhu, L.P.; Liao, G.H.; Bing, N.C.; Wang, L.L.; Yang, Y.; Xie, H.Y. Self-assembled 3D BiOCl hierarchitectures: Tunable synthesis and characterization. CrystEngComm 2010, 2, 3791–3796. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, X.; Chen, Y.; Huang, H.; Hu, J.; Wen, B. Fabrication of alveolate g-C3N4 with nitrogen vacancies via cobalt introduction for efficient photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2020, 45, 24792–24806. [Google Scholar] [CrossRef]
- Xu, S.; Carter, E.A. Theoretical insights into heterogeneous (photo) electrochemical CO2 reduction. Chem. Rev. 2019, 119, 6631–6669. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mensi, M.; Oveisi, E.; Mantella, V.; Buonsanti, R. Structural sensitivities in bimetallic catalysts for electrochemical CO2 reduction revealed by Ag-Cu nanodimers. J. Am. Chem. Soc. 2019, 141, 2490–2499. [Google Scholar] [CrossRef]
- Parvanian, A.M.; Sadeghi, N.; Rafiee, A.; Shearer, C.J.; Jafarian, M. Application of porous materials for CO2 reutilization: A review. Energies 2022, 15, 63. [Google Scholar] [CrossRef]
- Xie, C.; Niu, Z.; Kim, D.; Li, M.; Yang, P. Surface and interface control in nanoparticle catalysis. Chem. Rev. 2020, 120, 1184–1249. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous single-atom photocatalysts: Fundamentals and applications. Chem. Rev. 2020, 120, 12175–12216. [Google Scholar] [CrossRef]
- Wei, X.; Akbar, M.U.; Raza, A.; Li, G. A review on bismuth oxyhalide based materials for photocatalysis. Nanoscale Adv. 2021, 3, 3353–3372. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Amal, R.; Qiao, S.; Wu, J.; Yin, Z. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Y.; Luo, X.; Dan, Y.; Ye, J.; Zhou, Y.; Zou, Z. Beyond C3N4 π-conjugated metal-free polymeric semiconductors for photocatalytic chemical transformations. Chem. Soc. Rev. 2021, 50, 2147–2172. [Google Scholar] [CrossRef]
- Siao, C.W.; Chen, H.L.; Chen, L.W.; Chang, J.L.; Yeh, T.W.; Chen, C.C. Controlled hydrothermal synthesis of bismuth oxychloride-bismuth oxybromide-bismuth oxyiodide composites exhibiting visible-light photocatalytic degradation of 2-hydroxybenzoic acid and crystal violet. J. Colloid Interface Sci. 2018, 526, 322–336. [Google Scholar] [CrossRef]
- Li, K.L.; Lee, W.W.; Lu, C.S.; Dai, Y.M.; Chou, S.Y.; Chen, H.L.; Lin, H.P.; Chen, C.C. Synthesis of BiOBr, Bi3O4Br, and Bi12O17Br2 by controlled hydrothermal method and their photocatalytic properties. J. Taiwan Inst. Chem. Eng. 2014, 45, 2688–2697. [Google Scholar] [CrossRef]
- Jiang, Y.R.; Chou, S.Y.; Chang, J.L.; Huang, S.T.; Lin, H.P.; Chen, C.C. Hydrothermal synthesis of bismuth oxybromide–bismuth oxyiodide composites with high visible light photocatalytic performance for the degradation of CV and phenol. RSC Adv. 2015, 5, 30851–30860. [Google Scholar] [CrossRef]
- Liao, Y.H.B.; Wang, J.X.; Lin, J.S.; Chung, W.H.; Lin, W.Y.; Chen, C.C. Synthesis, photocatalytic activities and degradation mechanism of Bi2WO6 toward crystal violet dye. Catal. Today 2011, 174, 148–159. [Google Scholar] [CrossRef]
- Oppong, S.O.B.; Opoku, F.; Govender, P.P. Remarkable Enhancement of Eu–TiO2–GO Composite for Photodegradation of Indigo Carmine: A Design Method Based on Computational and Experimental Perspectives. Catal. Lett. 2020, 151, 1111–1126. [Google Scholar] [CrossRef]
- Chen, L.W.; Chen, H.L.; Lu, C.S.; Huang, S.T.; Yeh, T.W.; Chen, C.C. Preparation of perovskite-like PbBiO2I/g-C3N4 exhibiting visible-light-driven activity. Catal. Today 2021, 375, 472–483. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Vijayan, B.K.; Poluektov, O.G.; Rajh, T.; Gray, K.A.; He, H.; Zapol, P. Role of water and carbonates in photocatalytic transformation of CO2 to CH4 on titania. J. Am. Chem. Soc. 2011, 133, 3964–3971. [Google Scholar] [CrossRef]
- Gandhi, R.; Moses, A.; Sundar Baral, S. Fundamental study of the photocatalytic reduction of CO2: A short review of thermodynamics, kinetics and mechanisms. Chem. Process Eng. 2022, 43, 223–228. [Google Scholar]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Fan, H.J.; Lu, C.S.; Lee, W.L.; Chiou, M.R.; Chen, C.C. Mechanistic pathways differences between P25-TiO2 and Pt-TiO2 mediated CV photodegradation. J. Hazard. Mater. 2011, 185, 227–235. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jia, H.; Wamer, W.G.; Zheng, Z.; Li, P.; Callahan, J.H.; Yin, J.-J. Predicting and identifying reactive oxygen species and electrons for photocatalytic metal sulfide micro–nano structures. J. Catal. 2014, 320, 97–105. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Zhang, Z.; Wang, C.; Yang, Y.; Li, Q.; Xu, D. Lead-free perovskite Cs2AgBiBr6@g-C3N4 Z-scheme system for improving CH4 production in photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 282, 119570. [Google Scholar] [CrossRef]















| Molar Ratio (Cl:I = 1:2) | ||||
|---|---|---|---|---|
| pH | Temperature (°C) | |||
| 100 | 150 | 200 | 250 | |
| 1 | B1C1I2-1-100-12 | B1C1I2-1-150-12 | B1C1I2-1-200-12 | B1C1I2-1-250-12 |
| 4 | B1C1I2-4-100-12 | B1C1I2-4-150-12 | B1C1I2-4-200-12 | B1C1I2-4-250-12 |
| 7 | B1C1I2-7-100-12 | B1C1I2-7-150-12 | B1C1I2-7-200-12 | B1C1I2-7-250-12 |
| 10 | B1C1I2-10-100-12 | B1C1I2-10-150-12 | B1C1I2-10-200-12 | B1C1I2-10-250-12 |
| 13 | B1C1I2-13-100-12 | B1C1I2-13-150-12 | B1C1I2-13-200-12 | B1C1I2-13-250-12 |
| Molar Ratio (Cl:I = 2:1) | ||||
| pH | Temperature (°C) | |||
| 100 | 150 | 200 | 250 | |
| 1 | B1C2I1-1-100-12 | B1C2I1-1-150-12 | B1C2I1-1-200-12 | B1C2I1-1-250-12 |
| 4 | B1C2I1-4-100-12 | B1C2I1-4-150-12 | B1C2I1-4-200-12 | B1C2I1-4-250-12 |
| 7 | B1C2I1-7-100-12 | B1C2I1-7-150-12 | B1C2I1-7-200-12 | B1C2I1-7-250-12 |
| 10 | B1C2I1-10-100-12 | B1C2I1-10-150-12 | B1C2I1-10-200-12 | B1C2I1-10-250-12 |
| 13 | B1C2I1-13-100-12 | B1C2I1-13-150-12 | B1C2I1-13-200-12 | B1C2I1-13-250-12 |
| Cl I = 1 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | pH | |||||||||
| 1 | 4 | 7 | 10 | 13 | ||||||
| k (h−1) | R2 | k (h−1) | R2 | k (h−1) | R2 | k (h−1) | R2 | k (h−1) | R2 | |
| 100 | 0.01 | 0.974 | 0.034 | 0.982 | 0.027 | 0.986 | 0.043 | 0.977 | 0.008 | 0.903 |
| 150 | 0.021 | 0.939 | 0.036 | 0.977 | 0.026 | 0.986 | 0.025 | 0.974 | 0.008 | 0.903 |
| 200 | 0.005 | 0.812 | 0.033 | 0.984 | 0.031 | 0.979 | 0.035 | 0.986 | 0.013 | 0.994 |
| 250 | 0.017 | 0.973 | 0.068 | 0.983 | 0.035 | 0.945 | 0.033 | 0.961 | 0.015 | 0.997 |
| Photocatalyst | k (h−1) | R2 |
|---|---|---|
| BC2I1-100-4-g-C3N420% | 0.0208 | 0.8935 |
| BC2I1-100-4-g-C3N440% | 0.0349 | 0.92 |
| BC2I1-100-4-g-C3N460% | 0.0244 | 0.9958 |
| BC2I1-100-4-g-C3N480% | 0.0331 | 0.9843 |
| g-C3N4 | 0.0162 | 0.9027 |
| BC1I2-250-4-g-C3N420% | 0.2456 | 0.9732 |
| BC1I2-250-4-g-C3N440% | 0.1041 | 0.9767 |
| BC1I2-250-4-g-C3N460% | 0.1943 | 0.9938 |
| BC1I2-250-4-g-C3N480% | 0.0584 | 0.9887 |
| BC2I1-150-4-g-C3N420% | 0.0125 | 0.9027 |
| BC2I1-150-4-g-C3N440% | 0.0349 | 0.8125 |
| BC2I1-150-4-g-C3N460% | 0.0244 | 0.9841 |
| BC2I1-150-4-g-C3N480% | 0.0347 | 0.9786 |
| BC1I2-250-4-g-C3N40.5% | 0.0868 | 0.9859 |
| BC1I2-250-4-g-C3N41% | 0.1962 | 0.9421 |
| BC1I2-250-4-g-C3N42% | 0.1656 | 0.9896 |
| BC1I2-250-4-g-C3N45% | 0.0514 | 0.9849 |
| BC1I2-250-4-g-C3N410% | 0.0373 | 0.9339 |
| BC1I2-250-4-g-C3N412% | 0.0462 | 0.9811 |
| BC1I2-250-4-g-C3N415% | 0.0474 | 0.9565 |
| BC1I2-250-4-g-C3N418% | 0.0399 | 0.944 |
| BC1I2-250-4-g-C3N425% | 0.0492 | 0.9346 |
| BC1I2-250-4-g-C3N430% | 0.0462 | 0.9811 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.-M.; Wu, W.-T.; Lin, Y.-Y.; Wu, H.-L.; Chen, S.-H.; Jehng, J.-M.; Lin, J.-H.; Liu, F.-Y.; Chen, C.-C. Photocatalytic CO2 Reduction to CH4 and Dye Degradation Using Bismuth Oxychloride/Bismuth Oxyiodide/Graphitic Carbon Nitride (BiOmCln/BiOpIq/g-C3N4) Nanocomposite with Enhanced Visible-Light Photocatalytic Activity. Catalysts 2023, 13, 522. https://doi.org/10.3390/catal13030522
Dai Y-M, Wu W-T, Lin Y-Y, Wu H-L, Chen S-H, Jehng J-M, Lin J-H, Liu F-Y, Chen C-C. Photocatalytic CO2 Reduction to CH4 and Dye Degradation Using Bismuth Oxychloride/Bismuth Oxyiodide/Graphitic Carbon Nitride (BiOmCln/BiOpIq/g-C3N4) Nanocomposite with Enhanced Visible-Light Photocatalytic Activity. Catalysts. 2023; 13(3):522. https://doi.org/10.3390/catal13030522
Chicago/Turabian StyleDai, Yong-Ming, Wu-Tsan Wu, Yu-Yun Lin, Hsiao-Li Wu, Szu-Han Chen, Jih-Mirn Jehng, Jia-Hao Lin, Fu-Yu Liu, and Chiing-Chang Chen. 2023. "Photocatalytic CO2 Reduction to CH4 and Dye Degradation Using Bismuth Oxychloride/Bismuth Oxyiodide/Graphitic Carbon Nitride (BiOmCln/BiOpIq/g-C3N4) Nanocomposite with Enhanced Visible-Light Photocatalytic Activity" Catalysts 13, no. 3: 522. https://doi.org/10.3390/catal13030522
APA StyleDai, Y. -M., Wu, W. -T., Lin, Y. -Y., Wu, H. -L., Chen, S. -H., Jehng, J. -M., Lin, J. -H., Liu, F. -Y., & Chen, C. -C. (2023). Photocatalytic CO2 Reduction to CH4 and Dye Degradation Using Bismuth Oxychloride/Bismuth Oxyiodide/Graphitic Carbon Nitride (BiOmCln/BiOpIq/g-C3N4) Nanocomposite with Enhanced Visible-Light Photocatalytic Activity. Catalysts, 13(3), 522. https://doi.org/10.3390/catal13030522