ZnO/TiO2 Composite Thin-Film Photocatalysts for Gas-Phase Oxidation of Ethanol
Abstract
:1. Introduction
1.1. Photocatalysis
1.2. Comparison of TiO2 and ZnO Photocatalysts
1.3. Methods to Improve Photocatalytic Efficiency
1.4. Photocatalysis in Gas-Phase Applications
1.5. Photocatalytic Oxidation of Ethanol
2. Results
2.1. Photocatalyst Characterizations
2.1.1. X-ray Diffraction (XRD)
2.1.2. Scanning Electron Microscopy (SEM) and Energy Dispersion Spectrometry (EDS)
2.1.3. Bandgap Analyses
2.1.4. BET Surface Area
2.1.5. FTIR Analyses
2.2. Photocatalytic Performance for the Gas-Phase Oxidation of Ethanol
2.2.1. Adsorption of Ethanol Vapors and Initial Reactivity
2.2.2. Photocatalytic Oxidation of Ethanol after 24 h on Steam
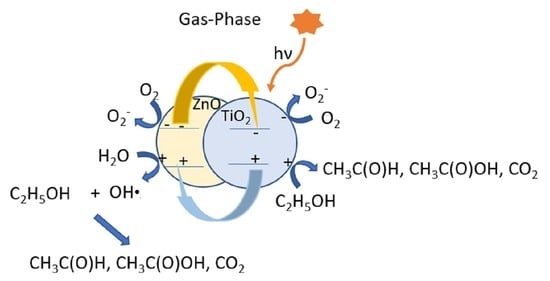
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Sol-Gel Synthesis of Pure and Composite TiO2 and ZnO Photocatalysts
4.3. Characterization Techniques
4.4. Photocatalytic Reactor and Test System
4.5. Photocatalytic Performance
4.5.1. Dark Adsorption and Initial Photocatalytic Activity
4.5.2. Photocatalytic Activity after 24 h of Continuous Reactor Operation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horikoshi, S.; Serpone, N. Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal. Today 2020, 340, 334–346. [Google Scholar] [CrossRef]
- Coronado, J.M. A Historical Introduction to Photocatalysis. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Green Energy and Technology; Coronado, J., Fresno, F., Hernández-Alonso, M., Portela, R., Eds.; Springer: London, UK, 2013. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to enhance ZnO photocatalyst’s performance for water treatment: A comprehensive review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.V.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Lin, L.; Chai, Y.; Zhao, B.; Wei, W.; He, D.; He, B.; Tang, Q. Photocatalytic oxidation for degradation of VOCs. Open J. Inorg. Chem. 2013, 3, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Alberici, R.M.; Jardim, W.F. Photocatalytic destruction of VOCs in the gas phase using titanium dioxide. Appl. Catal. B Environ. 1997, 14, 55–68. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile organic compounds (VOCs) removal by photocatalysts: A review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef] [PubMed]
- Almquist, C.B.; Kocher, J.; Saxton, K.; Simonson, L.; Danciutiu, A.; Nguyen, P.J.; Bain, J. A Novel Application of Photocatalysis: A UV-LED Photocatalytic Device for Controlling Diurnal Evaporative Fuel Vapor Emissions from Automobiles. Catalysts 2023, 13, 85. [Google Scholar] [CrossRef]
- Sahel, K.; Elsellami, L.; Mirali, I.; Dappozze, F.; Bouhent, M.; Guillard, C. Hydrogen peroxide and photocatalysis. Appl. Catal. B Environ. 2016, 188, 106–112. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2− and OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Domènech, X.; Ayllón, J.A.; Peral, J. H2O2 Formation from photocatalytic processes at the ZnO/water interface. Environ. Sci. Pollut. Res. 2001, 8, 285–287. [Google Scholar] [CrossRef]
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 photocatalysis of oxygenated and deoxygenated aqueous systems: A probe for photocatalytically produced hydroxyl radicals. J. Phys. Chem. C 2014, 118, 10083–10087. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Reconsideration of intrinsic band alignments within anatase and rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 431–434. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia Contributors. Direct and Indirect Band Gaps. Wikipedia, The Free Encyclopedia. Available online: https://en.wikipedia.org/w/index.php?title=Direct_and_indirect_band_gaps&oldid=1105981718 (accessed on 12 July 2023).
- Xu, Z.; Ren, Y.; Deng, X.; Xu, M.; Chai, W.; Qian, X.; Bian, Z. Recent Developments on Gas-Phase Volatile Organic Compounds Abatement Based on Photocatalysis. Adv. Energy Sustain. Res. 2022, 3, 2200105. [Google Scholar] [CrossRef]
- Bai, L.J.; Gang, K.O.U.; Gong, Z.Y.; Zhao, Z.M. Effect of Zn and Ti mole ratio on microstructure and photocatalytic properties of magnetron sputtered TiO2–ZnO heterogeneous composite film. Trans. Nonferrous Met. Soc. China 2013, 23, 3643–3649. [Google Scholar] [CrossRef]
- Serpone, N.A.V.E.; Emeline, A.V. Semiconductor Photocatalysis Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ortega, D.; Meléndez, A.M.; Acevedo-Peña, P.; González, I.; Arroyo, R. Semiconducting properties of ZnO/TiO2 composites by electrochemical measurements and their relationship with photocatalytic activity. Electrochim. Acta 2014, 140, 541–549. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A Chem. 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Krasteva, L.K.; Papazova, K.I.; Bojinova, A.S.; Kaneva, N.V.; Apostolov, A.A. Synthesis and characterization of ZnO and TIO2 powders, nanowire ZnO and TiO2/ZnO thin films for photocatalytic applications. Bulg. Chem. Commun. 2013, 45, 625–630. [Google Scholar]
- Rangkooy, H.A.; Jahani, F.; Siahi Ahangar, A. Photocatalytic removal of xylene as a pollutant in the air using ZnO-activated carbon, TiO2-activated carbon, and TiO2/ZnO activated carbon nanocomposites. Environ. Health Eng. Manag. J. 2020, 7, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Lachom, V.; Poolcharuansin, P.; Laokul, P. Preparation, Characterizations and Photocatalytic Activity of a ZnO/TiO2 Nanocomposite. Mater. Res. Express 2017, 4, 035006. Available online: https://iopscience.iop.org/article/10.1088/2053-1591/aa60d1 (accessed on 9 August 2023). [CrossRef]
- Mohsin, A.K. Preparation TiO2 and ZnO/TiO2 nanocomposites locally and use against Staphylococcus aureus. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 072014. [Google Scholar]
- Shalaby, A.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Dimitriev, Y.; Stoyanova, A.; Hitkova, H.; Ivanova, N.; Sredkova, M. Sol-gel synthesis and properties of nanocomposites in the Ag/TiO2/ZnO system. J. Optoelectroics Adv. Mater. 2015, 17, 248–256. [Google Scholar]
- Chen, J.; Liao, W.; Jiang, Y.; Yu, D.; Zou, M.; Zhu, H.; Zhang, M.; Du, M. Facile fabrication of ZnO/TiO2 heterogeneous nanofibres and their photocatalytic behaviour and mechanism towards rhodamine B. Nanomater. Nanotechnol. 2016, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Bai, N.; Liu, X.; Li, Z.; Ke, X.; Zhang, K.; Wu, Q. High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kuk, Y.S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and characterization of ZnO-TiO2/carbon fiber composite with enhanced photocatalytic properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.; Song, H.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L.P. Synthesis of ZnO: TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2019, 160, 154–163. [Google Scholar] [CrossRef]
- Hussein, A.M.; Mahoney, L.; Peng, R.; Kibombo, H.; Wu, C.M.; Koodali, R.T.; Shende, R. Mesoporous coupled ZnO/TiO2 photocatalyst nanocomposites for hydrogen generation. J. Renew. Sustain. Energy 2013, 5, 033118. [Google Scholar] [CrossRef]
- Li, D.; Jiang, X.; Zhang, Y.; Zhang, B.; Pan, C. A novel route to ZnO/TiO2 heterojunction composite fibers. J. Mater. Res. 2013, 28, 507–512. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chou, Y.Y.; Cheng, C.L.; Chen, Y.F. Giant enhancement of band edge emission based on ZnO/TiO2 nanocomposites. Opt. Express 2007, 15, 13832–13837. [Google Scholar] [CrossRef]
- Hellen, N.; Park, H.; Kim, K.N.; Hellen, N.; Park, H.; Kim, K.N. Characterization of ZnO/TiO2 nanocomposites prepared via the sol-gel method. J. Korean Ceram. Soc. 2018, 55, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Simsek, E.B.; Kilic, B.; Asgin, M.; Akan, A. Graphene oxide based heterojunction TiO2–ZnO catalysts with outstanding photocatalytic performance for bisphenol-A, ibuprofen and flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Mofokeng, S.J.; Kumar, V.; Kroon, R.E.; Ntwaeaborwa, O.M. Structure and optical properties of Dy3+ activated sol-gel ZnO-TiO2 nanocomposites. J. Alloys Compd. 2017, 711, 121–131. [Google Scholar] [CrossRef]
- Wang, F.; Yu, D.; Dai, J. Photoelectrochemical characteristics of ZnO/TiO2 nanoheterojunctions. AIP Adv. 2019, 9, 035237. [Google Scholar] [CrossRef]
- Upadhaya, D.; Kumar, P.; Dhar Purkayastha, D. Superhydrophobic ZnO/TiO2 Heterostructure with Significantly Enhanced Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2019, 30, 10399–10407. Available online: https://link.springer.com/article/10.1007/s10854-019-01381-2 (accessed on 9 August 2023). [CrossRef]
- Li, Y.; Wang, L.; Liang, J.; Gao, F.; Yin, K.; Dai, P. Hierarchical heterostructure of ZnO@TiO2 hollow spheres for highly efficient photocatalytic hydrogen evolution. Nanoscale Res. Lett. 2017, 12, 531. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Wang, D.; Wang, J.; Jiang, B.; Jiao, S.; Liu, D.; Zeng, Z.; Zhao, C.; Liu, Y.; et al. Efficient photocatalytic hydrogen evolution over TiO2-x mesoporous spheres-ZnO nanorods heterojunction. Nanomaterials 2020, 10, 2096. [Google Scholar] [CrossRef] [PubMed]
- Siuleiman, S.; Kaneva, N.; Bojinova, A.; Papazova, K.; Apostolov, A.; Dimitrov, D. Photodegradation of Orange II by ZnO and TiO2 powders and nanowire ZnO and ZnO/TiO2 thin films. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 408–413. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Morales-Luna, M.; Arvizua, M.A.; Tellez-Cruz, M.M. Optical, structural, and morphological properties of photocatalytic TiO2-ZnO thin films synthesized by the sol-gel process. Thin Solid Film. 2015, 594, 304–309. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, C.; Liu, Y.; Chen, H.; Li, H.; Wu, J. Comparison on photocatalytic degradation of gaseous formaldehyde by TiO2, ZnO and their composite. Ceram. Int. 2012, 38, 4437–4444. [Google Scholar] [CrossRef]
- Moradi, S.; Aberoomand-Azar, P.; Raeis-Farshid, S.; Abedini-Khorrami, S.; Givianrad, M.H. The effect of different molar ratios of ZnO on characterization and photocatalytic activity of TiO2/ZnO nanocomposite. J. Saudi Chem. Soc. 2016, 20, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Mediouni, N.; Dappozze, F.; Khrouz, L.; Parola, S.; Amara, A.B.H.; Rhaiem, H.B.; Jaffrezic-Renault, N.; Namour, P.; Guillard, C. Correlation between Photocatalytic Properties of ZnO and Generation of Hydrogen Peroxide—Impact of Composite ZnO/TiO2 Rutile and Anatase. Catalysts 2022, 12, 1445. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO binary oxide systems: Comprehensive characterization and tests of photocatalytic activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Štrbac, D.; Aggelopoulos, C.A.; Štrbac, G.; Dimitropoulos, M.; Novaković, M.; Ivetić, T.; Yannopoulos, S.N. Photocatalytic degradation of Naproxen and methylene blue: Comparison between ZnO, TiO2 and their mixture. Process Saf. Environ. Prot. 2018, 113, 174–183. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies–abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [Green Version]
- Jaison, A.; Mohan, A.; Lee, Y.C. Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts 2023, 13, 407. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; He, H. Photo-catalytic degradation of volatile organic compounds (VOCs) over titanium dioxide thin film. Adv. Asp. Spectrosc. 2012, 12, 341–372. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Rene, E.R.; Krishnan, J. Photocatalytic degradation of volatile pollutants. J. Environ. Chem. Toxicol. 2018, 2, 57–59. [Google Scholar]
- Liqiang, J.; Baifu, X.; Fulong, Y.; Baiqi, W.; Keying, S.; Weimin, C.; Honggang, F. Deactivation and regeneration of ZnO and TiO2 nanoparticles in the gas phase photocatalytic oxidation of n-C7H16 or SO2. Appl. Catal. A Gen. 2004, 275, 49–54. [Google Scholar] [CrossRef]
- Saucedo-Lucero, J.O.; Arriaga, S. Study of ZnO-photocatalyst deactivation during continuous degradation of n-hexane vapors. J. Photochem. Photobiol. A Chem. 2015, 312, 28–33. [Google Scholar] [CrossRef]
- Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic degradation of hexane vapors in batch and continuous systems using impregnated ZnO nanoparticles. Chem. Eng. J. 2013, 218, 358–367. [Google Scholar] [CrossRef]
- Muggli, D.S.; McCue, J.T.; Falconer, J.L. Mechanism of the photocatalytic oxidation of ethanol on TiO2. J. Catal. 1998, 173, 470–483. [Google Scholar] [CrossRef]
- Coronado, J.M.; Kataoka, S.; Tejedor-Tejedor, I.; Anderson, M.A. Dynamic phenomena during the photocatalytic oxidation of ethanol and acetone over nanocrystalline TiO2: Simultaneous FTIR analysis of gas and surface species. J. Catal. 2003, 219, 219–230. [Google Scholar] [CrossRef]
- Nimlos, M.R.; Wolfrum, E.J.; Brewer, M.L.; Fennell, J.A.; Bintner, G. Gas-phase heterogeneous photocatalytic oxidation of ethanol: Pathways and kinetic modeling. Environ. Sci. Technol. 1996, 30, 3102–3110. [Google Scholar] [CrossRef]
- Sola, A.C.; Garzón Sousa, D.; Araña, J.; González Díaz, O.; Doña Rodríguez, J.M.; Ramírez de la Piscina, P.; Homs, N. Differences in the vapour phase photocatalytic degradation of ammonia and ethanol in the presence of water as a function of TiO2 characteristics and the presence of O2. Catal. Today 2016, 266, 53–61. [Google Scholar] [CrossRef]
- Takeuchi, M.; Deguchi, J.; Sakai, S.; Anpo, M. Effect of H2O vapor addition on the photocatalytic oxidation of ethanol, acetaldehyde and acetic acid in the gas phase on TiO2 semiconductor powders. Appl. Catal. B Environ. 2010, 96, 218–223. [Google Scholar] [CrossRef]
- Sauer, M.L.; Ollis, D.F. Photocatalyzed oxidation of ethanol and acetaldehyde in humidified air. J. Catal. 1996, 158, 570–582. [Google Scholar] [CrossRef]
- Piera, E.; Ayllón, J.A.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Masschaele, K.; Moortgat, E.; Korany, T.E.; Hauchecorne, B.; Martens, J.A.; Lenaerts, S. Factors driving the activity of commercial titanium dioxide powders towards gas phase photocatalytic oxidation of acetaldehyde. Catal. Sci. Technol. 2012, 2, 2311–2318. [Google Scholar] [CrossRef]
- Almquist, C.B.; O’Hare, I.; Garza, L.; Badahman, A.; Jung, W.; Hanzel, S.; Neal, J. UV-LED Photocatalytic Device for the Oxidation of Ethanol and Hexane Vapors in Air. Chem. Proc. 2021, 6, 4. [Google Scholar] [CrossRef]
- Nargiello, M.; Herz, T. Physical-Chemical Characteristics of P-25 making it extremely suited as the catatalyst in photodegradation of organic compounds. Photocatalytic Purif. Treat. Water Air 1993, 3, 801–807. [Google Scholar]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Almquist, C.B.; Biswas, P. Role of Synthesis Method and Particle Size of Nanostructured TiO2 on Its Photoactivity. J. Catal. 2002, 212, 145–156. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles after Thermal Treatments and HF Etching. Front. Mater. 2020, 7, 192. Available online: https://www.frontiersin.org/articles/10.3389/fmats.2020.00192/full (accessed on 9 August 2023). [CrossRef]
- Han, E.; Vijayarangamuthu, K.; Youn, J.-S.; Park, Y.-K.; Jung, S.-C.; Jeon, K.-J. Degussa P25 TiO2 modified with H2O2 under microwave treatment to enhance photocatalytic properties. Catal. Today 2018, 303, 305–312. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- RealUV™ LED Strip Lights. Available online: https://store.waveformlighting.com/products/real-uv-led-strip-lights (accessed on 12 July 2023).
- RealUV™ LED Strip Lights Specification Sheet. Available online: https://store.waveformlighting.com/cdn/shop/files/realUV_LED_Strip_Lights_Specification_Sheet.pdf?v=2156247376753912624 (accessed on 12 July 2023).
- Lee, J.; Easteal, A.J.; Pal, U.; Bhattacharyya, D. Evolution of ZnO nanostructures in sol–gel synthesis. Curr. Appl. Phys. 2009, 9, 792–796. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.D.C. Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef] [Green Version]













| Catalyst ID | ZnO/TiO2 Mass Ratio a | Predominant Crystal Structure | Intended Mass Ratio (Zn/Ti) | Measured Mass Ratio (Zn/Ti) b | BET Surface Area (m2/g) | Crystal Size (nm) c | Bandgap (eV) |
|---|---|---|---|---|---|---|---|
| P25 | Anatase TiO2 | 49.5 | 22.9 | 3.05 | |||
| 100T0Z | 0 | Anatase TiO2 | 0 | 0 | 49.8 | 17.5 | 3.08 |
| 95T5Z | 0.05 | Anatase TiO2 | 0.07 | 0.085 | 37.7 | 14.2 | 3.19 |
| 90T10Z | 0.10 | Anatase TiO2 | 0.15 | 0.17 | 20.6 | 15.9 | 3.05 |
| 75T25Z | 0.25 | ZnTiO3 | 0.44 | 0.43 | 22.5 | 41.2 | 3.05 |
| 0T100Z | 1 | zincite | --- | --- | 9.15 | 40.6 | 3.18 |
| ZnO | zincite | 3.5 | 70.4 | 3.35 |
| Catalyst ID | k′ (µmoles/min/m2 Illuminated Area) | k″ (µmoles/min/m2 Surface Area) | Ke (µmoles/m3) | R2 |
|---|---|---|---|---|
| P25 | 4977 | 2.08 | 0.02 | 0.9981 |
| 100T0Z | 5006 | 2.08 | 0.02 | 0.9944 |
| 95T5Z | 562 | 0.308 | 0.21 | 0.9816 |
| 90T10Z | 106 | 0.127 | 1.3 | 0.9666 |
| 75T25Z | 104 | 0.096 | 2.6 | 0.9454 |
| 0T100Z | 13 | 0.036 | 2.1 | 1 1 |
| ZnO | 21 | 0.123 | 10 | 0.8967 |
| Gas-Phase Photocatalysis | |||
|---|---|---|---|
| Photocatalyst | Analyte | Conclusions | Reference |
| ZnO/TiO2 (Zn/Ti mass ratio = 0:1; 0.07:1; 0.15:1; 0.44:1; 1:0) | Ethanol (Ranging from 180–1800 ppm) | Pure TiO2 and P25 TiO2 had the highest apparent photocatalytic activity under UVA illumination in a flow reactor. As ZnO content in the composite materials increased, the apparent photocatalytic activity also decreased. The surface areas of the composite materials also decreased with increasing ZnO content. | This study |
| ZnO/Activated carbon; TiO2/Activated carbon ZnO/TiO2/Activated carbon | Xylene (100 ppm) | For up to 120 min of operation in a flow reactor, a composite 1ZnO/3TiO2/Activated carbon had a higher removal rate of xylene than AC/ZnO 5% and AC/TiO2 15%. | [30] |
| ZnO/TiO2 xZnO/(1 − x)TiO2 x = molar ratios: 1, 0.8, 0.6, 0.4, 0.2, 0 | Formaldehyde (50 ppm) | Pure TiO2 had the highest apparent photocatalytic activity under UVA illumination in a gas chamber. As ZnO content in the composite materials increased, the apparent photocatalytic activity decreased. The surface areas of the materials were not reported. | [52] |
| ZnO nanoparticles TiO2 nanoparticles | Heptane, SO2 | ZnO was more readily deactivated than TiO2 when oxidizing heptane under UVA illumination. Both ZnO and TiO2 were deactivated when oxidizing SO2. The surface areas of the materials were not reported. | [62] |
| ZnO on Perlite | Hexane (284 ppm) | ZnO was deactivated in less than 14 h in a flow reactor illuminated at 254 nm wavelength. It was concluded that water content on the photocatalyst was responsible for photocatalyst deactivation. | [63] |
| TiO2 on Perlite TiO2 on Poraver ZnO on Perlite ZnO on Poraver | Hexane (~500 ppm) | Similar hexane degradation rates for ZnO and TiO2 were observed in batch tests (254 nm wavelength). For continuous experiments, the TiO2 catalyst supported on Perlite showed higher degradation velocities than ZnO. However, when normalized to BET surface area, the ZnO impregnated onto Poraver resulted in better performance than TiO2 and ZnO on Perlite. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanusi, I.; Almquist, C.B. ZnO/TiO2 Composite Thin-Film Photocatalysts for Gas-Phase Oxidation of Ethanol. Catalysts 2023, 13, 1203. https://doi.org/10.3390/catal13081203
Sanusi I, Almquist CB. ZnO/TiO2 Composite Thin-Film Photocatalysts for Gas-Phase Oxidation of Ethanol. Catalysts. 2023; 13(8):1203. https://doi.org/10.3390/catal13081203
Chicago/Turabian StyleSanusi, Ibrahim, and Catherine B. Almquist. 2023. "ZnO/TiO2 Composite Thin-Film Photocatalysts for Gas-Phase Oxidation of Ethanol" Catalysts 13, no. 8: 1203. https://doi.org/10.3390/catal13081203
APA StyleSanusi, I., & Almquist, C. B. (2023). ZnO/TiO2 Composite Thin-Film Photocatalysts for Gas-Phase Oxidation of Ethanol. Catalysts, 13(8), 1203. https://doi.org/10.3390/catal13081203