Selective Production of Aromatics from 2-Octanol on Zinc Ion-Exchanged MFI Zeolite Catalysts
Abstract
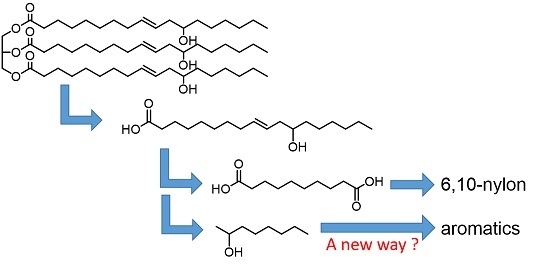
:1. Introduction
2. Results and Discussion
2.1. Carbon Balance of the Reaction and Stability of the Catalyst

2.2. Effects of Zeolite Structure and the Silica Alumina Ratio

2.3. Catalysis on Zinc Ion-Exchanged MFI Zeolites

2.4. Reaction Pathways and Reactivity of Various Compounds


3. Experimental Section
3.1. Preparation of Metal Ion-Exchanged Zeolite Catalysts
| Type of Zeolite | SiO2/Al2O3 Ratio (mol/mol) | Surface Area (m2/g, the BET Method) b | Crystal Size (μm) | NH3-TPD c (mmol/g) |
|---|---|---|---|---|
| Beta (BEA) | 150 | 560 | 0.7 | 0.6 |
| Faujasite (FAU) | 5.3 | 550 | 0.3 | 0.7 |
| L-type (LTL) | 6 | 290 | 0.4 | -d |
| ZSM-5 (MFI) | 33 | 330 | 0.1 × 0.5 | 1.8 |
| 40 | 330 | 2 × 4 | 1.3 | |
| 90 | 310 | 2 × 4 | 0.8 | |
| 1880 | 310 | 2 × 5 | -d | |
| Mordenite (MOR) | 90 | 400 | 0.1 × 0.5 | 1.2 |
3.2. Measurement of Catalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castor Oil Production, Extraction, Filtration, Purification&Refining. Available online: http://www.castoroil.in/extraction/extraction.html (accessed on 8 December 2015).
- Carothers, W.H. Synthetic Fiber. U.S. Patent 2130948, 20 September 1938. [Google Scholar]
- 2-Octanol. Available online: http://www.chemicalbook.com/ChemicalProductProperty_EN_CB3272125.htm (accessed on 8 December 2015).
- Caballero, A.; Perez, P.J. Methane as raw material in synthetic chemistry: The final frontier. Chem. Soc. Rev. 2013, 42, 8809–8820. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Shale Gas Revolution: An Opportunity for the Production of Biobased Chemicals? Angew. Chem. Inter. Ed. 2013, 52, 11980–11987. [Google Scholar] [CrossRef] [PubMed]
- Armor, J.N. Emerging importance of shale gas to both the energy & chemicals landscape. J. Energy Chem. 2013, 22, 21–26. [Google Scholar]
- Chang, C.D. Hydrocarbons from methanol. Catal. Rev. Sci. Eng. 1983, 25, 1–118. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Nayak, V.S. Conversion of alcohols to aromatics on H-SM-5: Influence of Si/Al ratio and degree of cation exchange on product distribution. Zeolites 1985, 5, 325–328. [Google Scholar] [CrossRef]
- Tynjälä, P.; Pakkanen, T.T.; Mustamäki, S. Modification of ZSM-5 Zeolite with Trimethyl Phosphite. 2. Catalytic Properties in the Conversion of C1–C4 Alcohols. J. Phys. Chem. B 1998, 102, 5280–5286. [Google Scholar] [CrossRef]
- Barthos, R.; Széchenyi, A.; Solymosi, F. Decomposition and Aromatization of Ethanol on ZSM-Based Catalysts. J. Phys. Chem. B 2006, 110, 21816–21825. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Sakuma, S.; Takeguchi, T.; Ueda, W. Direct Synthesis of n-Butanol from Ethanol over Nonstoichiometric Hydroxyapatite. Ind. Eng. Chem. Res. 2006, 45, 8634–8642. [Google Scholar] [CrossRef]
- Tsuchida, T.; Yoshikawa, T.; Sakuma, S.; Takeguchi, T.W.; Ueda, W. Synthesis of Biogasoline from Ethanol over Hydroxyapatite Catalyst. Ind. Eng. Chem. Res. 2008, 47, 1443–1452. [Google Scholar] [CrossRef]
- Mole, T.; Anderson, J.R. The reaction of propane over ZSM-5-H and ZSM-5-Zn zeolite catalysts. Appl. Catal. 1985, 17, 141–154. [Google Scholar] [CrossRef]
- Sirokman, G.; Sendoda, Y.; Ono, Y. Conversion of pentane into aromatics over ZSM-5 zeolites. Zeolites 1986, 6, 299–303. [Google Scholar] [CrossRef]
- Viswanadham, N.; Pradhan, A.R.; Ray, N.; Vishnoi, S.C.; Shanker, U.; Rao, T.P. Reaction pathways for the aromatization of paraffins in the presence of H-ZSM-5 and Zn/H-ZSM-5. Appl. Catal. A 1996, 137, 225–233. [Google Scholar] [CrossRef]
- Bendt, H.; Lietz, G.; Volter, J. Zinc promoted H-ZSM-5 catalysts for conversion of propane to aromatics II. Nature of the active sites and their activation. Appl. Catal. A 1996, 146, 365–379. [Google Scholar] [CrossRef]
- Lukyanov, D.B. Application of a kinetic model for investigation of aromatization reactions of light paraffins and olefins over HZSM-5. Stud. Surf. Sci. Catal. 1997, 105, 1301–1308. [Google Scholar]
- Biscardi, J.A.; Meitzner, G.D.; Iglesia, E. Structure and Density of Active Zn Species in Zn/H-ZSM5 Propane Aromatization Catalysts. J. Catal. 1998, 179, 192–202. [Google Scholar] [CrossRef]
- Pierella, L.B.; Eimer, G.A.; Anunziata, O.A. Selective ethane conversion into aromatic hydrocarbons over Zn-ZSM-11. React. Kinet. Catal. Lett. 1998, 63, 271–278. [Google Scholar] [CrossRef]
- Biscardi, J.A.; Iglesia, E. Reaction Pathways and Rate-Determining Steps in Reactions of Alkanes on H-ZSM5 and Zn/H-ZSM5 Catalysts. J. Catal. 1999, 182, 117–128. [Google Scholar] [CrossRef]
- Lusbango, L.M.; Scurrell, M.S. Light Alkanes aromatization to BTX over Zn-ZSM-5 catalysts: Enhancements in BTX selectivity by means of a second transition metal ion. Appl. Catal. A 2002, 235, 265–272. [Google Scholar] [CrossRef]
- Miyaji, A.; Sakamoto, Y.; Iwase, Y.; Yashima, T.; Koide, R.; Motokura, K.; Baba, T. Selective production of ethylene and propylene via monomolecular cracking of pentene over proton-exchanged zeolites: Pentene cracking mechanism determined by spatial volume of zeolite cavity. J. Catal. 2013, 302, 101–114. [Google Scholar] [CrossRef]
- Bernard, J.R. Hydrocarbons aromatization on platinum alkaline zeolites. In Proceedings of the 5th International Conference on Zeolites, Heyden, London, UK, 2–6 June 1980; pp. 686–693.
- Besoukhanova, C.; Guidot, J.; Barthomeuf, D.; Breysse, M.; Bernard, J.R. Platinum-zeolite interactions in alkaline L zeolites. Correlations between catalytic activity and platinum state. J. Chem. Soc. Faraday Trans. 1 1981, 77, 1595–1604. [Google Scholar] [CrossRef]
- Hughes, T.R.; Jacobson, R.L.; Tamm, P.W. Catalytic processes for octane enhancement by increasing the aromatics content of gasoline. Stud. Surf. Sci. Catal. 1988, 38, 317–333. [Google Scholar]
- Ostgard, D.J.; Kustov, L.; Poeppelmeier, K.R.; Sachtler, W.M.H. Comparison of Pt/KL catalysts prepared by ion exchange or incipient wetness impregnation. J. Catal. 1992, 133, 342–357. [Google Scholar] [CrossRef]
- Derouane, E.G.; Vanderveken, D. Structural recognition and preorganization in zeolite catalysis: Direct aromatization of n-hexane on zeolite L-based catalysts. Appl. Catal. 1988, 45, L15–L22. [Google Scholar] [CrossRef]
- Jongpatiwuta, S.; Trakarnroek, S.; Rirksomboon, T.; Osuwan, S.; Resasco, D.E. n-Octane aromatization on Pt-containing non-acidic large pore zeolite catalysts. Catal. Lett. 2005, 100, 7–15. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sawamura, N.; Iwamoto, M. Highly effective acetalization of aldehydes and ketones with methanol on siliceous mesoporous material. Tetrahedron Lett. 1998, 39, 9457–9460. [Google Scholar] [CrossRef]
- Ishitani, H.; Iwamoto, M. Selective aldol reactions of acetals on mesoporous silica catalyst. Tetrahedron Lett. 2003, 44, 299–301. [Google Scholar] [CrossRef]
- Iwamoto, M.; Tanaka, Y.; Sawamura, N.; Namba, S. Remarkable Effect of Pore Size on the Catalytic Activity of Mesoporous Silica for the Acetalization of Cyclohexanone with Methanol. J. Am. Chem. Soc. 2003, 125, 13032–13033. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, H.; Naito, H.; Iwamoto, M. Friedel-Crafts acylation of anisole with carboxylic anhydrides of large molecular sizes on mesoporous silica catalyst. Catal. Lett. 2008, 120, 14–18. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Selective synthesis of α-substituted β-keto esters from aldehydes and diazo esters on mesoporous silica catalysts. Tetrahedron Lett. 2008, 49, 4788–4791. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various Substituents on aluminum-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Ishitani, H.; Iwamoto, M. Highly ordered aluminum-planted mesoporous silica as active catalyst for Biginelli reaction and formyl C–H insertion reaction with diazo ester. Phys. Chem. Chem. Phys. 2010, 12, 14452–14455. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Kosugi, Y. Highly Selective Conversion of Ethene to Propene and Butenes on Nickel Ion-Loaded Mesoporous Silica Catalysts. J. Phys. Chem. C 2007, 111, 13–15. [Google Scholar] [CrossRef]
- Ikeda, K.; Kawamura, Y.; Yamamoto, T.; Iwamoto, M. Effectiveness of the template-ion exchange method for appearance of catalytic activity of Ni-MCM-41 for the ethene to propene reaction. Catal. Commun. 2008, 9, 106–110. [Google Scholar] [CrossRef]
- Haishi, T.; Kasai, K.; Iwamoto, M. Fast and Quantitative Dehydration of Lower Alcohols to Corresponding Olefins on Mesoporous Silica Catalyst. Chem. Lett. 2011, 40, 624–626. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kasai, K.; Haishi, T. Conversion of Ethanol into Polyolefin Building Blocks: Reaction Pathways on Nickel Ion-loaded Mesoporous Silica. ChemSusChem 2011, 4, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Kurosawa, M.; Tanaka, M.; Iwamoto, M. One-path and Selective Conversion of Ethanol to Propene on Scandium-modified Indium Oxide Catalysts. Chem. Lett. 2012, 41, 892–894. [Google Scholar] [CrossRef]
- Iwamoto, M.; Mizuno, S.; Tanaka, M. Direct and Selective Production of Propene from Bio-Ethanol on Sc-Loaded In2O3 Catalysts. Chem. Eur. J. 2013, 19, 7214–7220. [Google Scholar] [CrossRef] [PubMed]
- Nakasaka, Y.; Okamura, T.; Konno, H.; Tago, T.; Masuda, T. Crystal Size of MFI-type Zeolite for Catalytic Cracking of n-Hexane under Reaction-control Condition. Microporous Mesoporous Mater. 2013, 182, 244–249. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, M.; Takezawa, R.; Morimoto, M. Selective Production of Aromatics from 2-Octanol on Zinc Ion-Exchanged MFI Zeolite Catalysts. Catalysts 2015, 5, 2122-2133. https://doi.org/10.3390/catal5042122
Iwamoto M, Takezawa R, Morimoto M. Selective Production of Aromatics from 2-Octanol on Zinc Ion-Exchanged MFI Zeolite Catalysts. Catalysts. 2015; 5(4):2122-2133. https://doi.org/10.3390/catal5042122
Chicago/Turabian StyleIwamoto, Masakazu, Ryota Takezawa, and Masao Morimoto. 2015. "Selective Production of Aromatics from 2-Octanol on Zinc Ion-Exchanged MFI Zeolite Catalysts" Catalysts 5, no. 4: 2122-2133. https://doi.org/10.3390/catal5042122
APA StyleIwamoto, M., Takezawa, R., & Morimoto, M. (2015). Selective Production of Aromatics from 2-Octanol on Zinc Ion-Exchanged MFI Zeolite Catalysts. Catalysts, 5(4), 2122-2133. https://doi.org/10.3390/catal5042122