Hydrogen Evolution Reaction of γ-Mo0.5W0.5 C Achieved by High Pressure High Temperature Synthesis
Abstract
:1. Introduction
2. Results and Discussion
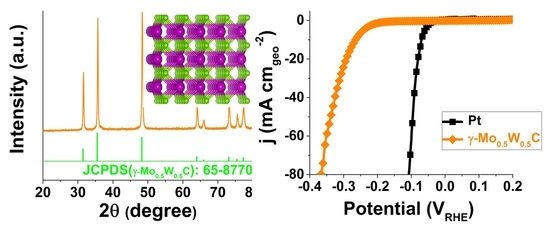
2.1. XRD Pattern and Raman Spectrum
2.2. XPS Analysis
2.3. SEM, TEM, HR-TEM, and SAED Images
2.4. Hydrogen Evolution Reaction of the γ-Mo0.5W0.5C Electrode
2.5. Durability and Stability Analysis
2.6. EIS Measurements
3. Experimental Section
3.1. Preparation of the γ-Mo0.5W0.5C Cathode
3.2. Electrochemical Measurement
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Luo, W.J.; Zhang, M.L.; Feng, J.Y.; Zou, Z.G. Photoelectrochemical cells for solar hydrogen production: Current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Li, Z.S.; Feng, J.Y.; Yan, S.C.; Zou, Z. Solar fuel production: Strategies and new opportunities with nanostructures. Nano Today 2015, 10, 468–486. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Wang, H.L.; Xie, L.M.; Liang, Y.Y.; Hong, G.S.; Dai, H.J. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hu, Y.F.; Qian, Q.F.; Yao, Y.F.; Zhang, S.Y.; Li, Z.S.; Zou, Z.G. Formation of hierarchical structure composed of (Co/Ni)Mn-LDH nanosheets on MWCNT backbones for efficient electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.F.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.S.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.T. Preparation and catalytic properties of transition metal carbides and nitrides. Catal. Today 1992, 15, 179–200. [Google Scholar] [CrossRef]
- Toth, L.E. Transition Metal Carbides and Nitrides; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Hwu, H.H.; Chen, J.G. Surface chemistry of transition metal carbides. Chem. Rev. 2005, 105, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.G.; Chen, J.G. Metal overlayer on metal carbide substrate: Unique bimetallic properties for catalysis and electrocatalysis. Chem. Soc. Rev. 2012, 41, 8021–8034. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rodriguez, J.A. Effects of carbon on the stability and chemical performance of transition metal carbides: A density functional study. J. Chem. Phy. 2004, 120, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- Vrubel, H.; Hu, X.L. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. 2012, 124, 12875–12878. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Roman-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.X.; Wu, H.B.; Xia, B.Y.; Xu, C.Y.; Lou, X.W. Hierarchical beta-Mo2C nanotubes organized by ultrathin nanosheets as a highly efficient electrocatalyst for hydrogen production. Angew. Chem. Int. Ed. 2015, 54, 15395–15399. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Hunt, S.T.; Nimmanwudipong, T.; Roman-Leshkov, Y. Engineering non–sintered, metal-terminated tungsten carbide nanoparticles for catalysis. Angew. Chem. Int. Ed. 2014, 53, 5131–5136. [Google Scholar]
- Wan, C.; Knight, N.A.; Leonard, B.M. Crystal structure and morphology control of molybdenum carbide nanomaterials synthesized from an amine-metal oxide composite. Chem. Commun. 2013, 49, 10409–10411. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. 2014, 126, 6525–6528. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kang, H.J.; Kim, D.J. Formation of TiC particle during carbothermal reduction of TiO2. J. Eur. Ceram. Soc. 2007, 27, 719–722. [Google Scholar] [CrossRef]
- Gogotsi, Y. Chemical vapour deposition: Transition metal carbides go 2D. Nat. Mater. 2015, 14, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Kakuta, T. MA-SHS of NbC and NbB2 in air from the Nb/B/C powder mixtures. J. Eur. Ceram. Soc. 2007, 27, 527–530. [Google Scholar] [CrossRef]
- Vallance, S.R.; Kingman, S.; Gregory, D.H. Ultra-rapid processing of refractory carbides; 20 s synthesis of molybdenum carbide, Mo2C. Chem. Commun. 2007, 38, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zheng, D.F.; Zhao, X.P.; Chen, Y.L.; Li, Q.; Li, Q.; Wang, C.C.; Cui, T.; Ma, Y.M.; Wang, X.; et al. Exploring Hardness and the Distorted sp2 Hybridization of B–B Bonds in WB3. Chem. Mater. 2014, 26, 5297–5302. [Google Scholar] [CrossRef]
- Luo, Y.B.; Yang, J.Y.; Li, G.; Liu, M.; Xiao, Y.; Fu, L.W.; Li, W.X.; Zhu, P.W.; Peng, J.Y.; Gao, S.; et al. Enhancement of the Thermoelectric Performance of Polycrystalline In4Se2.5 by Copper Intercalation and Bromine Substitution. Adv. Energy Mater. 2014, 4, 1300599. [Google Scholar] [CrossRef]
- Tao, Q.; Zhao, X.P.; Chen, Y.L.; Li, J.; Li, Q.; Ma, Y.M.; Li, J.J.; Cui, T.; Zhu, P.W.; Wang, X. Enhanced Vickers hardness by quasi-3D boron network in MoB2. RSC Adv. 2013, 3, 18317–18322. [Google Scholar] [CrossRef]
- Ma, S.L.; Bao, K.; Tao, Q.; Huang, X.L.; Zhu, P.W.; Cui, T. An ultra-incompressible ternary transition metal carbide. RSC Adv. 2014, 4, 63544–63548. [Google Scholar] [CrossRef]
- Xiao, P.; Ge, X.M.; Wang, H.B.; Liu, Z.L.; Fisher, A.; Wang, X. Novel Molybdenum carbide-tungsten carbide composite nanowires and their electrochemical activation for efficient and stable hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1520–1526. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.Q.; Cui, W.; Jiang, P.; Cheng, N.Y.; Asiri, A.M.; Sun, X.P. Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. 2014, 126, 6828–6832. [Google Scholar] [CrossRef]
- Kong, D.S.; Wang, H.T.; Lu, Z.Y.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.M.; Xu, Y.; Zhuo, S.F.; Zhang, J.F.; Zhang, B. Ni2P nanosheets/Ni foam composite electrode for long-lived and pH-tolerable electrochemical hydrogen generation. ACS Appl. Mater. Interfaces 2015, 7, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kolen’ko, Y.V.; Bao, X.Q.; Kovnir, K.; Liu, L. One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation. Angew. Chem. Int. Ed. 2015, 54, 8188–8192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Liu, Q.; Ge, C.J.; Cui, W.; Pu, Z.H.; Asiri, A.M.; Sun, X.P. CoP nanostructures with different morphologies: Synthesis, characterization and a study of their electrocatalytic performance toward the hydrogen evolution reaction. J. Mater. Chem. A 2014, 2, 14634–14640. [Google Scholar] [CrossRef]
- Xu, Y.F.; Gao, M.R.; Zheng, Y.R.; Jiang, J.; Yu, S.H. Nickel/Nickel(II) Oxide Nanoparticles Anchored onto Cobalt(IV) Diselenide Nanobelts for the Electrochemical Production of Hydrogen. Angew. Chem. Int. Ed. 2013, 52, 8546–8550. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Jia, G.; Ma, S.; Hu, J.; Zhu, P.; Cui, T.; Li, Z.; Zou, Z. Hydrogen Evolution Reaction of γ-Mo0.5W0.5 C Achieved by High Pressure High Temperature Synthesis. Catalysts 2016, 6, 208. https://doi.org/10.3390/catal6120208
Hu Y, Jia G, Ma S, Hu J, Zhu P, Cui T, Li Z, Zou Z. Hydrogen Evolution Reaction of γ-Mo0.5W0.5 C Achieved by High Pressure High Temperature Synthesis. Catalysts. 2016; 6(12):208. https://doi.org/10.3390/catal6120208
Chicago/Turabian StyleHu, Yingfei, Gan Jia, Shuailing Ma, Jianqiang Hu, Pinwen Zhu, Tian Cui, Zhaosheng Li, and Zhigang Zou. 2016. "Hydrogen Evolution Reaction of γ-Mo0.5W0.5 C Achieved by High Pressure High Temperature Synthesis" Catalysts 6, no. 12: 208. https://doi.org/10.3390/catal6120208
APA StyleHu, Y., Jia, G., Ma, S., Hu, J., Zhu, P., Cui, T., Li, Z., & Zou, Z. (2016). Hydrogen Evolution Reaction of γ-Mo0.5W0.5 C Achieved by High Pressure High Temperature Synthesis. Catalysts, 6(12), 208. https://doi.org/10.3390/catal6120208