Hydroprocessing of Oleic Acid for Production of Jet-Fuel Range Hydrocarbons over Cu and FeCu Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. N2- Adsorption/Desorption Measurement
2.2. XRD Analysis
2.3. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) and CO Chemisorption Analyses
2.4. Fourier Transform Infra-Red Analysis
2.5. XPS
2.6. H2-TPR Analysis
2.7. Pyridine FTIR Analysis
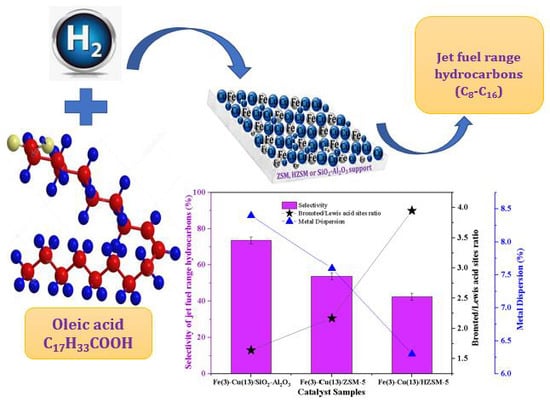
2.8. Catalyst Evaluation
3. Experimental Methods
3.1. Catalyst Synthesis
3.1.1. Chemical and Materials
3.1.2. Support Preparation
3.1.3. Catalyst Preparation by Impregnation
3.2. Catalyst Characterization
3.2.1. ICP-OES
3.2.2. N2-Adsorption/Desorption Measurement
3.2.3. XRD
3.2.4. XPS
3.2.5. FTIR Spectroscopy.
3.2.6. CO Chemisorption
3.2.7. Temperature Programmed Reduction (TPR)
3.2.8. Pyridine FTIR
3.3. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sust. Energ. Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef] [Green Version]
- Kieckhäfer, K.; Quante, G.; Müller, C.; Spengler, T.S.; Lossau, M.; Jonas, W. Simulation-Based Analysis of the Potential of Alternative Fuels towards Reducing CO2 Emissions from Aviation. Simulation-Based Analysis of the Potential of Alternative Fuels towards Reducing CO2 Emissions from Aviation. Energies 2018, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Bwapwaa, J.K.; Anandraj, A.; Trois, C. Possibilities for conversion of microalgae oil into aviation fuel: A review. Renew. Sust. Energ. Rev. 2017, 80, 1345–1354. [Google Scholar] [CrossRef]
- Sgouridis, S.; Bonnefoy, P.A.; Hansman, R.J. Air transportation in a carbon constrained world: Long-term dynamics of policies and strategies for mitigating the carbon footprint of commercial aviation. Transp. Res. Part A 2011, 45, 1077–1091. [Google Scholar] [CrossRef]
- Diederichs, G.W.; Mandegari, M.A.; Farzad, S.; Görgens, J.F. Techno-economic comparison of biojet fuel production from lignocellulose, vegetable oil and sugar cane juice. Bioresour. Technol. 2016, 216, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bykova, M.V.; Yu, D.; Ermakov, V.V.; Kaichev, O.A.; Bulavchenko, A.A.; Saraev, M.; Yu, L.; Yakovlev, V.A. Ni-based sol–gel catalysts as promising systems for crude bio-oil upgrading: Guaiacol hydrodeoxygenation study. Appl. Catal. B Environ. 2012, 113, 296–307. [Google Scholar] [CrossRef]
- Kandel, K.; Anderegg, J.W.; Nelson, N.C.; Chaudhary, U.; Slowing, I.I. Supported iron nanoparticles for the hydrodeoxygenation of microalgal oil to green diesel. J. Catal. 2014, 314, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Rabaev, M.; Landau, M.V.; Vidruk-Nehemya, R.; Koukouliev, V.; Zarchin, R.; Herskowitz, M. Conversion of vegetable oils on Pt/Al2O3/SAPO-11 to diesel and jet fuels containing aromatics. Fuel 2015, 161, 287–294. [Google Scholar] [CrossRef]
- Deepak, V.; Rohit, K.; Bharat, S.R.; Anil, K.S. Aviation fuel production from lipids by a single-step route using hierarchical mesoporous zeolites. Energ. Environ. Sci. 2011, 4, 1667. [Google Scholar] [CrossRef]
- Verma, D.; Rana, B.S.; Sibi, M.G.; Sinha, A.K. Diesel and aviation kerosene with desired aromatics from hydroprocessing of jatropha oil over hydrogenation catalysts supported on hierarchical mesoporous SAPO-11. Appl. Catal. A Gen. 2015, 490, 108–116. [Google Scholar] [CrossRef]
- Bennici, S.; Gervasini, A.; Ravasio, N.; Zaccheria, F. Optimization of Tailoring of CuOx Species of Silica Alumina Supported Catalysts for the Selective Catalytic Reduction of NOx. J. Phys. Chem. B 2003, 107, 5168–5176. [Google Scholar] [CrossRef]
- Kazuhisa, M.; Yanyong, L.; Megumu, I.; Isao, T. Production of Synthetic Diesel by Hydrotreatment of Jatropha Oils Using Pt-Re/H-ZSM-5 Catalyst. Energy Fuel 2010, 24, 2404–2409. [Google Scholar]
- Yanyong, L.; Rogelio, S.; Kazuhisa, M.; Tomoaki, M.; Kinya, S. Hydrotreatment of Vegetable Oils to Produce Bio-Hydrogenated Diesel and Liquefied Petroleum Gas Fuel over Catalysts Containing Sulfided NiMo and Solid Acids. Energy Fuel 2011, 25, 4675–4685. [Google Scholar]
- Ali, M.A.; Tatsumi, T.; Masuda, T. Development of heavy oil hydrocracking catalysts using amorphous silica-alumina and zeolites as catalyst supports. Appl. Catal. A Gen. 2002, 233, 77–90. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Cat. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Toyama, N.; Inoue, N.; Ohki, S.; Tansho, M.; Shimizu, T.; Umegaki, T.; Kojima, Y. Influence of hollow silica-alumina composite spheres prepared using various amount of L(+)-arginine on their activity for hydrolytic dehydrogenation of ammonia borane. Adv. Mater. Lett. 2016, 7, 339–343. [Google Scholar] [CrossRef]
- Saber, O.; Gobara, H.M. Optimization of silica content in alumina-silica nanocomposites to achieve high catalytic dehydrogenation activity of supported Pt catalyst. Egypt. J. Petrol. 2014, 23, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, N.H.; Elsalamony, R.A.; El Naggar, A.M.A. Mesoporous waste-extracted SiO2–Al2O3-supported Ni and Ni–H3PW12O40 nano-catalysts for photo-degradation of methyl orange dye under UV irradiation. New J. Chem. 2018, 42, 9177–9186. [Google Scholar] [CrossRef]
- Morettia, G.; Dossib, C.; Fusib, A.; Recchiab, S.; Psarob, R. A comparison between Cu-ZSM-5, Cu/S-1 and Cu/mesoporous silica alumina as catalysts for NO decomposition. Appl. Catal. B Environ. 1999, 20, 67–73. [Google Scholar] [CrossRef]
- Ishihara, A.; Negura, H.; Hashimoto, T.; Nasu, H. Catalytic properties of amorphous silica-alumina prepared using malic acid as a matrix in catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2010, 388, 68–76. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Liang, K.; Chang, S.H.; Tseng, Z.; Yeh, S.; Chen, C.; Wu, W.; Wu, C. Nano-structured CuO-Cu2O Complex Thin Film for Application in CH3NH3PbI3 Perovskite Solar Cells. Nanoscale Res. Lett. 2016, 11, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Kanoh, H.; Kaneko, K. ZSM-5 Monolith of Uniform Mesoporous Channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.Z.; Mauricio, J.E.; Paredes, A.R.; Gamero, P.; Cortés, D. Antimicrobial properties of ZSM-5 type zeolite functionalized with silver. Mater. Lett. 2017, 191, 65–68. [Google Scholar] [CrossRef]
- Betancourt-Galindo, R.; Reyes-Rodriguez, P.Y.; Puente-Urbina, B.A.; Avila-Orta, C.A.; Rodríguez-Fernández, O.S.; Cadenas-Pliego, G.; García-Cerda, L.A. Synthesis of Copper Nanoparticles by Thermal Decomposition and Their Antimicrobial Properties. J. Nanomater. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour, M.; Golzary, A.; Saber, M.; Yoshikawa, K. Denitrogenation of biocrude oil from algal biomass in high temperature water and formic acid mixture over HZSM-5 nanocatalyst. Fuel 2017, 206, 628–637. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Laua, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Biesingera, M.C.; Laua, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Feng, X.; Cao, Y.; Lan, L.; Lin, C.; Li, Y.; Xu, H.; Gong, M.; Chen, Y. The promotional effect of Ce on CuFe/beta monolith catalyst for selective catalytic reduction of NOx by ammonia. Chem. Eng. J. 2016, 302, 697–706. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, Y.; Liu, J.; Huang, Q.; He, S.; Li, C.; Zhao, J.; Wei, M. Catalytic conversion of syngas to mixed alcohols over CuFe-based catalysts derived from layered double hydroxides. Catal. Sci. Technol. 2013, 3, 1324. [Google Scholar] [CrossRef]
- He, Q.; Yang, X.; He, R.; Bueno-López, A.; Miller, H.; Ren, X.; Yang, W.; Koel, B.E. Electrochemical and spectroscopic study of novel Cu and Fe-based catalysts for oxygen reduction in alkaline media. J. Power Sources 2012, 213, 169–179. [Google Scholar] [CrossRef]
- He, Q.; Yang, X.; Ren, X.; Bruce, E.; Koel, B.E.; Ramaswamy, N.; Mukerjee, S.; Kostecki, R. A novel CuFe-based catalyst for the oxygen reduction reaction in alkaline media. J. Power Sources 2011, 196, 7404–7410. [Google Scholar] [CrossRef]
- Wang, M.; Shu, Z.; Zhang, L.; Fan, X.; Tao, G.; Wang, Y.; Chen, L.; Wu, M.; Shi, J. Amorphous Fe2+-rich FeOx loaded in mesoporous silica as a highly efficient heterogeneous Fenton catalyst. Dalton Trans. 2014, 43, 9234. [Google Scholar] [CrossRef]
- Xiao, Z.; Jin, S.; Wang, X.; Li, W.; Wang, J.; Liang, C. Preparation, structure and catalytic properties of magnetically separable Cu–Fe catalysts for glycerol hydrogenolysis. J. Mater. Chem. 2012, 22, 16598. [Google Scholar] [CrossRef]
- Manikandan, M.; Venugopal, A.K.; Nagpure, A.S.; Chilukuri, S.; Raja, T. Promotional effect of Fe on the performance of supported Cu catalyst for ambient pressure hydrogenation of furfural. RSC Adv. 2016, 6, 3888. [Google Scholar] [CrossRef]
- Sheng, H.; Lobo, R.F. Iron-Promotion of Silica-Supported Copper Catalysts for Furfural Hydrodeoxygenation. ChemCatChem 2016, 8, 3402–3408. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forn, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, L.; Song, Y.; Shang, Y.; Zhai, Y.; Gong, Y. A comparative synthesis of ZSM-5 with ethanol or TPABr template: Distinction of Brønsted/Lewis acidity ratio and its impact on n-hexane cracking. Catal. Sci. Technol. 2018, 8, 1923. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 5th ed.; Pearson Education Incorporation: Upper Saddle River, NJ, USA, 2016; pp. 734–735. [Google Scholar]
- Jothimurugesan, K.; Gangwal, S.K. Titania-Supported Bimetallic Catalysts Combined with HZSM-5 for Fischer-Tropsch Synthesis. Ind. Eng. Chem. Res. 1998, 37, 1181–1188. [Google Scholar] [CrossRef]
- Platon, A.; Thomson, W.J. Quantitative Lewis/Bro1nsted Ratios Using DRIFTS. Ind. Eng. Chem. Res. 2003, 24, 24. [Google Scholar] [CrossRef]
- Li, D.; Bui, P.; Zhao, H.Y.; Oyama, S.T.; Dou, T.; Shen, Z.H. Rake mechanism for the deoxygenation of ethanol over a supported Ni2P/SiO2 catalyst. J. Catal. 2012, 290, 1–12. [Google Scholar] [CrossRef]











| Sample ID | BET Surface Area (m2/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) | Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| SiO2-Al2O3 ZSM HZSM Cu(5)/SiO2-Al2O3 Cu(10)/SiO2-Al2O3 Cu(13)/SiO2-Al2O3 Cu(15)/SiO2-Al2O3 Fe(1)-Cu(13)/SiO2-Al2O3 Fe(2)-Cu(13)/SiO2-Al2O3 Fe(3)-Cu(13)//SiO2-Al2O3 Fe(5)-Cu(13)/SiO2-Al2O3 Fe(3)-Cu(13)/ZSM-5 Fe(3)-Cu(13)/HZSM-5 | 660 393 321 623 611 455 510 458 483 446 430 266 193 | 0.00 0.17 0.12 - - - - - - - - 0.10 0.09 | 0.94 0.07 0.08 0.94 0.81 0.81 0.60 0.58 0.59 0.50 0.47 0.06 0.01 | 0.94 0.24 0.20 0.94 0.81 0.80 0.60 0.58 0.59 0.50 0.47 0.16 0.10 | 5.7 2.4 2.5 5.2 5.2 5.3 5.0 5.1 4.9 4.5 4.4 2.4 2.1 | - - - 7.3 7.6 8.7 8.7 24.0 18.1 5.9 8.3 33.8 29.1 |
| Catalyst Samples | ICP-OES | Metals Dispersion (%) | Metallic Surface Area (m2/g Sample) | Metallic Surface Area (m2/g of Metal) | Metals Crystallite Size (nm) | |
|---|---|---|---|---|---|---|
| Cu (wt%) | Fe (wt%) | |||||
| Fe(3)-Cu(13)/SiO2-Al2O3 Fe(3)-Cu(13)/ZSM-5 Fe(3)-Cu(13)/HZSM-5 | 13.0 13.1 13.4 | 2.5 3.1 3.2 | 8.4 7.6 6.3 | 8.1 7.6 6.5 | 54.3 46.7 38.4 | 8.4 9.8 11.9 |
| Catalysts | Component | Binding Energy of Cu or Fe 2p3/2 (eV) | Atomic Composition (%) |
|---|---|---|---|
| Fe(3)-Cu(13)/HZSM | Cu2O Cu CuO Fe FeO Fe2O3 | 935 936 937 708 709 713 | 24.3 47.4 28.3 2.4 38.1 58.5 |
| Fe(3)-Cu(13)/SiO2-Al2O3 | Cu2O Cu CuO Fe FeO Fe2O3 | 935 936 937 708 709 713 | 57.1 1.7 41.2 25.9 35.6 38.5 |
| Fe(3)-Cu(13)/ZSM | Cu2O Cu CuO Fe FeO Fe2O3 | 935 936 937 708 709 713 | 31.4 52.9 15.7 15.5 33.3 51.2 |
| Catalyst Samples | Reduction Peak Temperature (°C) | H2 Uptake (mmol g−1 cat) |
|---|---|---|
| Fe(3)-Cu(13)/SiO2-Al2O3 Fe(3)-Cu(13)/ZSM Fe(3)-Cu(13)/HZSM | 207 289 321 | 3.1 2.6 1.6 |
| Catalyst | Temperature (°C) | Residence Time (h) | ||||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | ||
| Fe(3)-Cu(13)/SiO2-Al2O3 | 300 | 26.3 | 36.3 | 43.5 | 59.5 | 59.7 |
| 320 | 21.5 | 39.0 | 48.4 | 53.7 | 41.8 | |
| 340 | 45.4 | 45.3 | 45.2 | 45.2 | 51.8 | |
| Fe(3)-Cu(13)/ ZSM | 300 | 27.3 | 38.4 | 23.3 | 50.2 | 39.1 |
| 320 | 22.3 | 44.6 | 56.4 | 55.7 | 32.4 | |
| 340 | 47.0 | 40.0 | 49.9 | 56.4 | 52.5 | |
| Fe(3)-Cu(13)/ HZSM-5 | 300 | 4.6 | 8.5 | 14.7 | 38.0 | 30.1 |
| 320 | 19.2 | 29.5 | 38.3 | 39.5 | 38.9 | |
| 340 | 39.6 | 48.0 | 42.64 | 40.0 | 39.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayandiran, A.A.; Boahene, P.E.; Dalai, A.K.; Hu, Y. Hydroprocessing of Oleic Acid for Production of Jet-Fuel Range Hydrocarbons over Cu and FeCu Catalysts. Catalysts 2019, 9, 1051. https://doi.org/10.3390/catal9121051
Ayandiran AA, Boahene PE, Dalai AK, Hu Y. Hydroprocessing of Oleic Acid for Production of Jet-Fuel Range Hydrocarbons over Cu and FeCu Catalysts. Catalysts. 2019; 9(12):1051. https://doi.org/10.3390/catal9121051
Chicago/Turabian StyleAyandiran, Afees A., Philip E. Boahene, Ajay K. Dalai, and Yongfeng Hu. 2019. "Hydroprocessing of Oleic Acid for Production of Jet-Fuel Range Hydrocarbons over Cu and FeCu Catalysts" Catalysts 9, no. 12: 1051. https://doi.org/10.3390/catal9121051
APA StyleAyandiran, A. A., Boahene, P. E., Dalai, A. K., & Hu, Y. (2019). Hydroprocessing of Oleic Acid for Production of Jet-Fuel Range Hydrocarbons over Cu and FeCu Catalysts. Catalysts, 9(12), 1051. https://doi.org/10.3390/catal9121051