Electrospun Nanofibers Embedding ZnO/Ag2CO3/Ag2O Heterojunction Photocatalyst with Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Electrospun Nanofiber
2.1.1. XRD Analysis
2.1.2. Morphological Structure Analysis
2.1.3. Surface Area and Pore Size Distribution
2.1.4. FTIR Analysis
2.1.5. Optical Properties
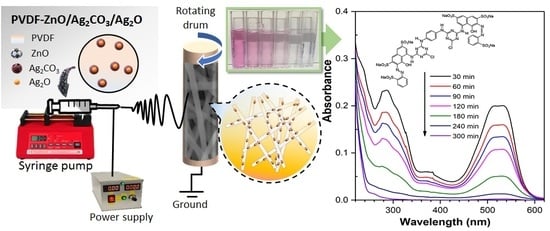
2.2. Evaluation of Photocatalytic Activity
3. Materials and Methods
3.1. Material
3.2. Preparation of the Electrospun Nanofiber
3.3. Characterization
3.4. Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting–a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and Bandgap Engineering Using ZnO Nanocomposites. Adv. Mater. Sci. Eng. 2015, 15, 1–22. [Google Scholar] [CrossRef]
- Song, L.; Qiu, R.; Mo, Y.; Zhang, D.; Wei, H.; Xiong, Y. Photodegradation of phenol in a polymer-modified TiO2 semiconductor particulate system under the irradiation of visible light. Catal. Commun. 2007, 9, 429–433. [Google Scholar] [CrossRef]
- Yan, Y.; Guan, H.; Liu, S.; Jiang, R. Ag3PO4/Fe2O3 composite photocatalysts with an n–n heterojunction semiconductor structure under visible-light irradiation. Ceram. Int. 2014, 40, 9095–9100. [Google Scholar] [CrossRef]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy Associates Promoting Solar-Driven Photocatalytic Activity of Ultrathin Bismuth Oxychloride Nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Sarsari, I.A.; Kameli, P.; Salamati, H. Effect of Ag doping on structural, optical, and photocatalytic properties of ZnO nanoparticles. J. Alloy. Compd. 2015, 640, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Feng, N.; Wang, Q.; Zheng, A.; Zhang, Z.; Fan, J.; Liu, S.B.; Amoureux, J.P.; Deng, F. Understanding the High Photocatalytic Activity of (B, Ag)-Codoped TiO2 under Solar-Light Irradiation with XPS, Solid-State NMR, and DFT Calculations. J. Am. Chem. Soc. 2013, 135, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, Ö.A.; Unalan, H.E.; Durucan, C. Highly Efficient Room Temperature Synthesis of Silver-Doped Zinc Oxide (ZnO:Ag) Nanoparticles: Structural, Optical, and Photocatalytic Properties. J. Am. Ceram. Soc. 2013, 96, 766–773. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.; Jaafar, J.; Rosmi, M.S.; Hir, Z.A.; Mutalib, M.A.; Ismail, A.F.; Tanemura, M. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO2: Bio-template assisted sol-gel synthesis and photocatalytic activity. Appl. Surf. Sci. 2017, 393, 46–59. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Abd Mutalib, M.; Jamil, S.M. Incorporation of N-doped TiO2 nanorods in regenerated cellulose thin films fabricated from recycled newspaper as a green portable photocatalyst. Carbohydr. Polym. 2015, 133, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pulgarin, C. Photocatalytic activity of N, S co-doped and N-doped commercial anatase TiO2 powders towards phenol oxidation and E. coli inactivation under simulated solar light irradiation. Sol. Energy 2010, 84, 37–43. [Google Scholar] [CrossRef]
- Yu, S.; Yun, H.J.; Kim, Y.H.; Yi, J. Carbon-doped TiO2 nanoparticles wrapped with nanographene as a high performance photocatalyst for phenol degradation under visible light irradiation. Appl. Catal. B Environ. 2014, 144, 893–899. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Hao, R.; Wang, L.; Shen, X.; Yu, R.; Limpanart, S.; Ma, M.; Liu, R. Carbon-Doped ZnO Nanostructures: Facile Synthesis and Visible Light Photocatalytic Applications. J. Phys. Chem. C 2015, 119, 20544–20554. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n–p–n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Zhang, L.; Xue, H.; Wang, C.; Liu, Y. Electrospun Nanofibers of ZnO-SnO2 Heterojunction with High Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Jaafar, J.; Zain, M.; Minggu, L.J.; Kassim, M.B.; Salehmin, M.N.I.; Rosmi, M.S.; Salleh, W.; Othman, M.H.D. Concurrent growth, structural and photocatalytic properties of hybridized C, N co-doped TiO2 mixed phase over g-C3N4 nanostructured. Scr. Mater. 2018, 142, 143–147. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Ternary ZnO/AgBr/Ag2CrO4 nanocomposites with tandem n–n heterojunctions as novel visible-light-driven photocatalysts with excellent activity. Ceram. Int. 2015, 41, 14383–14393. [Google Scholar] [CrossRef]
- Kiantazh, F.; Habibi-Yangjeh, A. Ag3VO4-ZnO nanocomposites with an n–n heterojunction as novel visible-light-driven photocatalysts with highly enhanced activity. Mater. Sci. Semicond. Process. 2015, 39, 671–679. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.; Ismail, A.F.; Jaafar, J.; Harun, Z.; Aziz, F.; Mohamed, M.A.; Ohtani, B.; Takashima, M. Photocatalytic degradation of phenol over visible light active ZnO/Ag2CO3/Ag2O nanocomposites heterojunction. J. Photochem. Photobiol. A Chem. 2018, 364, 602–612. [Google Scholar] [CrossRef]
- Ardoña, H.A.M.; Paredes, F.U.; Arellano, I.H.J.; Arco, S.D. Electrospun PET supported-ionic liquid-stabilized CdS catalyst for the photodegradation of Rhodamine B under visible light. Mater. Lett. 2013, 91, 96–99. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.; Mohamed, M.A.; Jaafar, J.; Ismail, A.; Harun, Z. Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interface Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.K. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal Nitrogen-Doped TiO2 Photocatalytic Coatings for Sunlight-Activated Membranes. Adv. Sustain. Syst. 2017, 1, 1600041. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Newnes: Oxford, UK, 2010; Volume 1. [Google Scholar]
- Rao, K.V.S.; Subrahmanyam, M.; Boule, P. Immobilized TiO2 photocatalyst during long-term use: Decrease of its activity. Appl. Catal. B Environ. 2004, 49, 239–249. [Google Scholar] [CrossRef]
- Hoogesteijn von Reitzenstein, N.; Bi, X.; Yang, Y.; Hristovski, K.; Westerhoff, P. Morphology, structure, and properties of metal oxide/polymer nanocomposite electrospun mats. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Optimization and an insightful properties—activity study of electrospun TiO2/CuO composite nanofibers for efficient photocatalytic H2 generation. Appl. Catal. B Environ. 2013, 140, 68–81. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.-c.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2 (B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Ali, M.E.M.; Österlund, L.; Khattab, I.A.; Badawy, M.I.; Farah, J.Y.; Zaher, F.M.; Al-Maghrabi, M.N. ZnO/spiral-shaped glass for solar photocatalytic oxidation of Reactive Red 120. Arab. J. Chem. 2017, 10, S3501–S3507. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Jiang, W.; Bechelany, M.; Nasr, M.; Jarvis, J.; Schaub, T.; Sapkota, R.R.; Miele, P.; Wang, H.; Xu, P. Adsorption and photocatalytic oxidation of ibuprofen using nanocomposites of TiO2 nanofibers combined with BN nanosheets: Degradation products and mechanisms. Chemosphere 2019, 220, 921–929. [Google Scholar] [CrossRef]
- Karim, S.A.; Mohamed, A.; Abdel-Mottaleb, M.; Osman, T.; Khattab, A. Visible light photocatalytic activity of PAN-CNTs/ZnO-NH2 electrospun nanofibers. J. Alloy. Compd. 2019, 772, 650–655. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.; Khalil, A.; Karim, S.; Osman, T.; Khattab, A. High performance of PAN/GO-ZnO composite nanofibers for photocatalytic degradation under visible irradiation. J. Mech. Behav. Biomed. 2019, 96, 118–124. [Google Scholar] [CrossRef]
- Mao, Z.; Xie, R.; Fu, D.; Zhang, L.; Xu, H.; Zhong, Y.; Sui, X. PAN supported Ag-AgBr@Bi20TiO32 electrospun fiber mats with efficient visible light photocatalytic activity and antibacterial capability. Sep. Purif. Technol. 2017, 176, 277–286. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nnao Lett. 2006, 4. [Google Scholar] [CrossRef]
- Patel, A.C.; Li, S.; Wang, C.; Zhang, W.; Wei, Y. Electrospinning of Porous Silica Nanofibers Containing Silver Nanoparticles for Catalytic Applications. Chem. Mater. 2007, 19, 1231–1238. [Google Scholar] [CrossRef]
- Wang, G.; Pan, C.; Wang, L.; Dong, Q.; Yu, C.; Zhao, Z.; Qiu, J. Activated carbon nanofiber webs made by electrospinning for capacitive deionization. Electrochim. Acta 2012, 69, 65–70. [Google Scholar] [CrossRef]
- Azad, A.-M. Fabrication of transparent alumina (Al2O3) nanofibers by electrospinning. Mater. Sci. Eng. A 2006, 435–436, 468–473. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Teng, H. Overview of the Development of the Fluoropolymer Industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- He, T.; Zhou, Z.; Xu, W.; Ren, F.; Ma, H.; Wang, J. Preparation and photocatalysis of TiO2–fluoropolymer electrospun fiber nanocomposites. Polymer 2009, 50, 3031–3036. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, X.; Yang, H.; Xu, Z.L.; Wei, Y.M. Preparation of Porous PVDF Nanofiber Coated with Ag NPs for Photocatalysis Application. Fibers Polym. 2016, 17, 21–29. [Google Scholar] [CrossRef]
- Richard, M.; Nthumbi, J.C.N. Electrospun and functionalized PVDF/PAN nanocatalyst-loaded composite for dechlorination and photodegradation of pesticides in contaminated water. Environ. Sci. Pollut. Res. 2016, 23, 20214–20231. [Google Scholar] [CrossRef]
- Brahma, S.; Rao, K.J.; Shivashankar, S. Rapid growth of nanotubes and nanorods of würtzite ZnO through microwave-irradiation of a metalorganic complex of zinc and a surfactant in solution. Bull. Mater. Sci. 2010, 33, 89–95. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Dai, G.; Hou, Q. Fabrication of Ag2CO3/SrCO3 Rods with Highly Efficient Visible-light Photocatalytic Activity. Rare Met. Mater. Eng. 2017, 46, 312–316. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a New Visible-Light Photocatalyst: Self-Stability and High Photocatalytic Activity. Chem. A Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, G.; Kumar, S.; Yang, K.; Jin, R. Phase Transformation Synthesis of Novel Ag2O/Ag2CO3 Heterostructures with High Visible Light Efficiency in Photocatalytic Degradation of Pollutants. Adv. Mater. 2014, 26, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Drew, C.; Wang, X.; Samuelson, L.A.; Kumar, J. The effect of viscosity and filler on electrospun fiber morphology. J. Macromol. Sci. Part A 2003, 40, 1415–1422. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. Effects of working parameters on electrospinning. In One-Dimensional Nanostructures; Springer: Berlin, Germany, 2013; pp. 15–28. [Google Scholar]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Ashraf, M.; Campagne, C.; Perwuelz, A.; Champagne, P.; Leriche, A.; Courtois, C. Development of superhydrophilic and superhydrophobic polyester fabric by growing zinc oxide nanorods. J. Colloid Interface Sci. 2013, 394, 545–553. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- ALOthman, Z. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Chen, S.; Tao, S.; Tang, D.; Xu, H.; Li, S.; Zhao, J.; Jiang, Q.; Yang, H. Pore structure characterization of different rank coals using N2 and CO2 adsorption and its effect on CH4 adsorption capacity: A case in Panguan syncline, Western Guizhou, china. Energy Fuels 2017, 31, 6034–6044. [Google Scholar] [CrossRef]
- Betz, N.; Le Moel, A.; Balanzat, E.; Ramillon, J.; Lamotte, J.; Gallas, J.; Jaskierowicz, G. A FTIR study of PVDF irradiated by means of swift heavy ions. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1493–1502. [Google Scholar] [CrossRef]
- Devi, P.I.; Ramachandran, K. Dielectric studies on hybridised PVDF–ZnO nanocomposites. J. Exp. Nanosci. 2011, 6, 281–293. [Google Scholar] [CrossRef]
- Li, C.; Xin, Q.; Guo, X.-X. Surface oxygen species and their reactivities in the mild oxidation of ethylene on cerium oxide studied by FT-IR spectroscopy. Catal. Lett. 1992, 12, 297–305. [Google Scholar] [CrossRef]
- Kadam, A.; Dhabbe, R.; Gophane, A.; Sathe, T.; Garadkar, K. Template free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orange. J. Photochem. Photobiol. B Biol. 2016, 154, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lamba, R.; Umar, A.; Mehta, S.; Kansal, S.K. Enhanced visible light driven photocatalytic application of Ag2O decorated ZnO nanorods heterostructures. Sep. Purif. Technol. 2017, 183, 341–349. [Google Scholar] [CrossRef]
- Yu, C.; Wei, L.; Chen, J.; Xie, Y.; Zhou, W.; Fan, Q. Enhancing the photocatalytic performance of commercial TiO2 crystals by coupling with trace narrow-band-gap Ag2CO3. Ind. Eng. Chem. Res. 2014, 53, 5759–5766. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Kupková, J.; Kustrowski, P.; Praus, P. Photocatalytic decomposition of N2O over TiO2/g-C3N4 photocatalysts heterojunction. Appl. Surf. Sci. 2017, 396, 1685–1695. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Huang, B.; Li, H.; Qin, X.; Zhang, X.; Dai, Y. Preparation, characterization, and photocatalytic properties of silver carbonate. Appl. Surf. Sci. 2011, 257, 8732–8736. [Google Scholar] [CrossRef]
- Almasian, A.; Chizari Fard, G.; Parvinzadeh Gashti, M.; Mirjalili, M.; Mokhtari Shourijeh, Z. Surface modification of electrospun PAN nanofibers by amine compounds for adsorption of anionic dyes. Desalin. Water Treat. 2016, 57, 10333–10348. [Google Scholar] [CrossRef]
- Makaremi, M.; De Silva, R.T.; Pasbakhsh, P. Electrospun nanofibrous membranes of polyacrylonitrile/halloysite with superior water filtration ability. J. Phys. Chem. C 2015, 119, 7949–7958. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Rivera, K.; Neramittagapong, A.; Wantala, K. Effects of hydrothermal temperature and time on uncalcined TiO2 synthesis for reactive red 120 photocatalytic degradation. Surf. Coat. Technol. 2015, 271, 192–200. [Google Scholar] [CrossRef]
- Panakoulias, T.; Kalatzis, P.; Kalderis, D.; Katsaounis, A. Electrochemical degradation of Reactive Red 120 using DSA and BDD anodes. J. Appl. Electrochem. 2010, 40, 1759–1765. [Google Scholar] [CrossRef]
- Balachandran, S.; Selvam, K.; Babu, B.; Swaminathan, M. The simple hydrothermal synthesis of Ag–ZnO–SnO2 nanochain and its multiple applications. Dalton Trans. 2013, 42, 16365–16374. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Cossich, E.; Bergamasco, R.; de Amorim, M.P.; Martins, P.; Marques, J.; Tavares, C.J.; Lanceros-Méndez, S.; Sencadas, V. Development of electrospun photocatalytic TiO2-polyamide-12 nanocomposites. Mater. Chem. Phys. 2015, 164, 91–97. [Google Scholar] [CrossRef]
- Dilpazir, S.; Usman, M.; Rasul, S.; Arshad, S. A simple UV-ozone surface treatment to enhance photocatalytic performance of TiO2 loaded polymer nanofiber membranes. RSC Adv. 2016, 6, 14751–14755. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Zain, M.F.; Minggu, L.J.; Kassim, M.B.; Amin, N.A.; Salleh, W.N.; Salehmin, M.N.; Nasir, M.F.; Hir, Z.A. Constructing bio-templated 3D porous microtubular C-doped g-C3N4 with tunable band structure and enhanced charge carrier separation. Appl. Catal. B Environ. 2018, 236, 265–279. [Google Scholar] [CrossRef]
- Paul, J.; Rawat, K.P.; Sarma, K.S.; Sabharwal, S. Decoloration and degradation of Reactive Red-120 dye by electron beam irradiation in aqueous solution. Appl. Radiat. Isot. 2011, 69, 982–987. [Google Scholar] [CrossRef] [PubMed]










| Samples | BET Surface Area (m2g−1) | Average Pore Size (nm) | Pore Volume (×10−2 cm3g−1) |
|---|---|---|---|
| P0 | 3.75 | 23.97 | 2.94 |
| P1 | 4.12 | 22.47 | 2.31 |
| P2 | 6.61 | 13.86 | 2.29 |
| P3 | 6.50 | 11.26 | 1.83 |
| P4 | 6.22 | 18.91 | 2.80 |
| Electrospun Nanofiber | Sample |
|---|---|
| PVDF | P0 |
| PVDF-ZnO | P1 |
| PVDF-ZnO/Ag2CO3 | P2 |
| PVDF-ZnO/Ag2CO3/Ag2O | P3 |
| PVDF-ZnO/Ag2O | P4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosman, N.; Wan Salleh, W.N.; Aziz, F.; Ismail, A.F.; Harun, Z.; Bahri, S.S.; Nagai, K. Electrospun Nanofibers Embedding ZnO/Ag2CO3/Ag2O Heterojunction Photocatalyst with Enhanced Photocatalytic Activity. Catalysts 2019, 9, 565. https://doi.org/10.3390/catal9070565
Rosman N, Wan Salleh WN, Aziz F, Ismail AF, Harun Z, Bahri SS, Nagai K. Electrospun Nanofibers Embedding ZnO/Ag2CO3/Ag2O Heterojunction Photocatalyst with Enhanced Photocatalytic Activity. Catalysts. 2019; 9(7):565. https://doi.org/10.3390/catal9070565
Chicago/Turabian StyleRosman, Nurafiqah, Wan Norharyati Wan Salleh, Farhana Aziz, Ahmad Fauzi Ismail, Zawati Harun, Syamsutajri Syamsol Bahri, and Kazukiyo Nagai. 2019. "Electrospun Nanofibers Embedding ZnO/Ag2CO3/Ag2O Heterojunction Photocatalyst with Enhanced Photocatalytic Activity" Catalysts 9, no. 7: 565. https://doi.org/10.3390/catal9070565
APA StyleRosman, N., Wan Salleh, W. N., Aziz, F., Ismail, A. F., Harun, Z., Bahri, S. S., & Nagai, K. (2019). Electrospun Nanofibers Embedding ZnO/Ag2CO3/Ag2O Heterojunction Photocatalyst with Enhanced Photocatalytic Activity. Catalysts, 9(7), 565. https://doi.org/10.3390/catal9070565