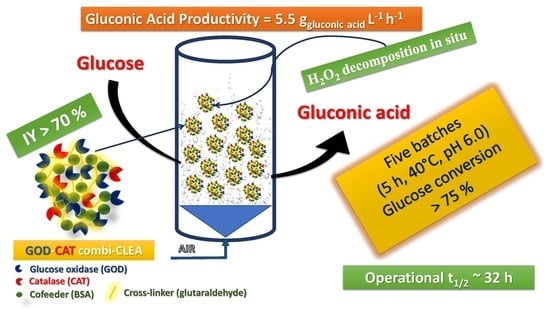
Combi-CLEAs of Glucose Oxidase and Catalase for Conversion of Glucose to Gluconic Acid Eliminating the Hydrogen Peroxide to Maintain Enzyme Activity in a Bubble Column Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Conditions for Preparing GOD-CAT Combi-CLEAs
2.1.1. Selection of the Precipitant Agent
2.1.2. Selection of Crosslinking Conditions
2.1.3. Study of BSA as a Protein Feeder on the Preparation of GOD/CAT Combi-CLEAs
2.1.4. Thermo-Stability of Combi-CLEAs
2.1.5. Surface Morphology and Size Particle Characterization
2.2. GA Production
2.3. Reusability
2.4. Operational Half-Life
3. Material and Methods
3.1. Material
3.2. GOD and CAT Activity Assays
3.3. Protein Concentration
3.4. Combi-CLEA Preparation
3.5. Stability of Combi-CLEAs
3.6. Gluconic Acid Production and Operational Stability
3.7. Apparent Kinetic Parameters of Combi-CLEAs Using Glucose Modification
3.8. SEM Images
3.9. Chromatographic Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


| Assay | BSA (mg/mL) | BSA/Enzymes Ratio (w/w) | [GLU] mM | μmol GLU/mgprot. | IY (%) | Observed Activity (U mL−1) | Recovered Activity (%) | Observed Activity after Stability Assay (U mL−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 25 | 4.55 | 29.7 ± 2.7 | 355.5 ± 32.0 | 8.0 ± 0.9 | 96.1 ± 10.6 |
| 2 | 0 | 0 | 50 | 9.09 | 42.3 ± 3.8 | 505.9 ± 45.5 | 16.0 ± 1.8 | 190.9 ± 21.0 |
| 3 | 0 | 0 | 100 | 18.18 | 41.2 ± 3.7 | 492.2 ± 44.3 | 10.8 ± 1.2 | 129.6 ± 14.3 |
| 4 | 0 | 0 | 200 | 36.36 | 24.2 ± 2.2 | 289.9 ± 26.1 | 5.2 ± 0.6 | 62.1 ± 6.8 |
| 5 | 15 | 1.36 | 25 | 1.92 | 59.4 ± 5.4 | 711.0 ± 64.0 | 39.6 ± 4.4 | 474.0 ± 52.1 |
| 6 | 15 | 1.36 | 50 | 3.85 | 55.8 ± 5.0 | 667.2 ± 60.0 | 41.8 ± 4.6 | 500.4 ± 55.0 |
| 7 | 15 | 1.36 | 100 | 7.69 | 50.8 ± 4.6 | 607.1 ± 54.6 | 30.4 ± 3.3 | 364.2 ± 40.1 |
| 8 | 15 | 1.36 | 200 | 15.38 | 45.7 ± 4.1 | 546.9 ± 49.2 | 34.3 ± 3.8 | 410.2 ± 45.1 |
| 9 | 30 | 2.73 | 25 | 1.22 | 59.4 ± 5.4 | 711.0 ± 64.0 | 54.6 ± 6.0 | 652.8 ± 71.8 |
| 10 | 30 | 2.73 | 50 | 2.44 | 32.0 ± 2.9 | 382.8 ± 34.5 | 24.0 ± 2.6 | 287.1 ± 31.6 |
| 11 | 30 | 2.73 | 100 | 4.88 | 32.0 ± 2.9 | 382.8 ± 34.5 | 25.3 ± 2.8 | 303.0 ± 33.3 |
| 12 | 30 | 2.73 | 200 | 9.76 | 19.2 ± 1.7 | 229.7 ± 20.7 | 19.2 ± 2.1 | 229.7 ± 25.3 |
| 13 | 60 | 5.45 | 25 | 0.70 | 70.9 ± 6.4 | 847.7 ± 76.3 | 53.2 ± 5.8 | 635.8 ± 69.9 |
| 14 | 60 | 5.45 | 50 | 1.41 | 42.8 ± 3.8 | 511.3 ± 46.0 | 34.2 ± 3.8 | 409.1 ± 45.0 |
| 15 | 60 | 5.45 | 100 | 2.82 | 44.4 ± 4.0 | 530.5 ± 47.7 | 43.4 ± 4.8 | 519.3 ± 57.1 |
| 16 | 60 | 5.45 | 200 | 5.63 | 34.5 ± 3.1 | 412.3 ± 37.1 | 32.2 ± 3.5 | 385.6 ± 42.4 |
| Assay | BSA (mg/mL) | BSA/Enzymes Ratio (w/w) | [GLU] mM | μmol GLU/mgprot. | IY (%) | Observed Activity (U mL−1) | Recovered Activity (%) | Observed Activity after Stability Assay (U mL−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 25 | 4.55 | 42.2 ± 4.6 | 3320.6 ± 365.3 | 42.2 ± 5.0 | 3320.6 ± 395.2 |
| 2 | 0 | 0 | 50 | 9.09 | 48.4 ± 4.3 | 3806.6 ± 342.4 | 39.5 ± 5.8 | 3112.6 ± 453.0 |
| 3 | 0 | 0 | 100 | 18.18 | 10.3 ± 1.1 | 813.1 ± 89.4 | 10.3 ± 1.2 | 813.1 ± 96.8 |
| 4 | 0 | 0 | 200 | 36.36 | 6.8 ± 0.7 | 533.1 ± 58.6 | 6.8 ± 0.8 | 533.1 ± 63.4 |
| 5 | 15 | 1.36 | 25 | 1.92 | 48.4 ± 5.0 | 3812.6 ± 397.8 | 45.9 ± 5.8 | 3616.3 ± 453.7 |
| 6 | 15 | 1.36 | 50 | 3.85 | 50.2 ± 4.2 | 3950.6 ± 330.9 | 38.2 ± 6.0 | 3008.2 ± 470.1 |
| 7 | 15 | 1.36 | 100 | 7.69 | 51.5 ± 5.7 | 4053.1 ± 445.8 | 51.5 ± 6.1 | 4053.1 ± 482.3 |
| 8 | 15 | 1.36 | 200 | 15.38 | 40.6 ± 2.9 | 3192.2 ± 229.4 | 26.5 ± 4.8 | 2085.6 ± 379.9 |
| 9 | 30 | 2.73 | 25 | 1.22 | 62.7 ± 4.1 | 4933.7 ± 326.5 | 37.7 ± 7.5 | 2967.8 ± 587.1 |
| 10 | 30 | 2.73 | 50 | 2.44 | 46.7 ± 3.6 | 3675.2 ± 284.6 | 32.9 ± 5.6 | 2586.9 ± 437.3 |
| 11 | 30 | 2.73 | 100 | 4.88 | 27.0 ± 2.7 | 2125.4 ± 211.4 | 24.4 ± 3.2 | 1921.5 ± 252.9 |
| 12 | 30 | 2.73 | 200 | 9.76 | 24.7 ± 2.1 | 1947.0 ± 165.5 | 19.1 ± 2.9 | 1504.3 ± 231.7 |
| 13 | 60 | 5.45 | 25 | 0.70 | 80.4 ± 7.0 | 6329.2 ± 547.5 | 63.2 ± 9.6 | 4977.6 ± 753.2 |
| 14 | 60 | 5.45 | 50 | 1.41 | 78.9 ± 6.2 | 6206.5 ± 487.7 | 56.3 ± 9.4 | 4433.3 ± 738.6 |
| 15 | 60 | 5.45 | 100 | 2.82 | 68.9 ± 5.4 | 5424.6 ± 423.3 | 48.9 ± 8.2 | 3848.5 ± 645.5 |
| 16 | 60 | 5.45 | 200 | 5.63 | 39.1 ± 3.8 | 3080.3 ± 296.3 | 34.2 ± 4.7 | 2694.1 ± 366.6 |
References
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Singh, O.V.; Kumar, R. Biotechnological production of gluconic acid: Future implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Purane, N.K.; Sharma, S.K.; Salunkhe, P.D.; Labade, D.S.; Tondilikar, M.M. Gluconic acid production from golden syrup by Aspergillus niger strain using semiautomatic stirred-tank fermenter. J. Microb. Biochem. Technol. 2012, 4, 92–95. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Furlan, F.F.; Badino, A.C.; Tardioli, P.W. Gluconic acid production from sucrose in an airlift reactor using a multi-enzyme system. Bioprocess Biosyst. Eng. 2015, 38, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Godjevargova, T.; Dayal, R.; Turmanova, S. Gluconic acid production in bioreactor with immobilized glucose oxidase plus catalase on polymer membrane adjacent to anion-exchange membrane. Macromol. Biosci. 2004, 4, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Tomotani, E.J.; Vitolo, M. Invertase, glucose oxidase and catalase for converting sucrose to fructose and gluconic acid through batch and membranecontinuous reactors. Braz. J. Pharm. Sci. 2011, 47, 399–408. [Google Scholar] [CrossRef]
- Nakao, K.; Kiefner, A.; Furumoto, K.; Harada, T. Production of gluconic acid with immobilized glucose oxidase in airlift reactors. Chem. Eng. Sci. 1997, 52, 4127–4133. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Witt, S.; Wohlfahrt, G.; Schomburg, D.; Hecht, H.J.; Kalisz, H.M. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of beta-d-glucose. Biochem. J. 2000, 347, 553–559. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivić, S.; Wohlfahrt, G.; Kandrač, J.; Peričin, D. Glucose oxidase from Aspergillus niger: The mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int. J. Biochem. Cell Biol. 2005, 37, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Hydrogen peroxide in biocatalysis. A dangerous liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Godjevargova, T.; Dayal, R.; Marinov, I. Simultaneous covalent immobilization of glucose oxidase and catalase onto chemically modified acrylonitrile copolymer membranes. J. Appl. Polym. Sci. 2004, 91, 4057–4063. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; Giordano, R.L.C.; Ribeiro, M.P.A.; Tardioli, P.W.; de Lima Camargo Giordano, R.; de Arruda Ribeiro, M.P.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Xue, R.; Woodley, J.M. Process technology for multi-enzymatic reaction systems. Bioresour. Technol. 2012, 115, 183–195. [Google Scholar] [CrossRef]
- Ba, S.; Haroune, L.; Cruz-Morató, C.; Jacquet, C.; Touahar, I.E.; Bellenger, J.-P.; Legault, C.Y.; Jones, J.P.; Cabana, H. Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters. Sci. Total Environ. 2014, 487, 748–755. [Google Scholar] [CrossRef]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.-P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cui, C.; Chen, H.; Chen, B.; Tan, T. Genipin cross-linked glucose oxidase and catalase multi-enzyme for gluconic acid synthesis. Appl. Biochem. Biotechnol. 2017, 181, 526–535. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, L.; Ma, L.; He, Y.; Gao, J.; Li, D.; Jiang, Y. Co-immobilization of glucose oxidase and catalase in silica inverse opals for glucose removal from commercial isomaltooligosaccharide. Int. J. Biol. Macromol. 2018, 107, 2034–2043. [Google Scholar] [CrossRef]
- Cui, C.; Fang, Y.; Chen, B.; Tan, T. Glucose oxidation performance is improved by the use of a supramolecular self-assembly of glucose oxidase and catalase. Catal. Sci. Technol. 2019, 9, 477–482. [Google Scholar] [CrossRef]
- Nakao, K.; Harada, T.; Furumoto, K.; Kiefner, A.; Popovic, M. Mass transfer properties of bubble columns suspending immobilized glucose oxidase gel beads for gluconic acid production. Can. J. Chem. Eng. 1999, 77, 816–825. [Google Scholar] [CrossRef]
- Nakao, K.; Bao, J.; Harada, T.; Yasuda, Y.; Furumoto, K. Measurement and prediction of axial distribution of immobilized glucose oxidase gel beads suspended in bubble column. J. Chem. Eng. Jpn. 2000, 33, 721–729. [Google Scholar] [CrossRef]
- Bao, J.; Furumoto, K.; Fukunaga, K.; Nakao, K. A kinetic study on air oxidation of glucose catalyzed by immobilized glucose oxidase for production of calcium gluconate. Biochem. Eng. J. 2001, 8, 91–102. [Google Scholar] [CrossRef]
- Bao, J.; Koumatsu, K.; Arimatsu, Y.; Furumoto, K. A kinetic study on crystallization of calcium gluconate in external loop airlift column and stirred tank for an immobilized glucose oxidase reaction with crystallization. Biochem. Eng. J. 2003, 15, 177–184. [Google Scholar] [CrossRef]
- Bao, J.; Furumoto, K.; Fukunaga, K.; Nakao, K. Average and local oxygen transfer properties in bubble column with axial distribution of immobilized glucose oxidase gel beads. Chem. Eng. Sci. 2000, 55, 5405–5414. [Google Scholar] [CrossRef]
- Bao, J.; Koumatsu, K.; Furumoto, K.; Yoshimoto, M.; Fukunaga, K. Optimal operation of an integrated bioreaction—Crystallization process for continuous production of calcium gluconate using external loop airlift columns. Chem. Eng. Sci. 2001, 56, 6165–6170. [Google Scholar] [CrossRef]
- Zhuang, W.; Huang, J.; Liu, X.; Ge, L.; Niu, H.; Wang, Z.; Wu, J.; Yang, P.; Chen, Y.; Ying, H. Co-localization of glucose oxidase and catalase enabled by a self-assembly approach: Matching between molecular dimensions and hierarchical pore sizes. Food Chem. 2019, 275, 197–205. [Google Scholar] [CrossRef]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; John Wiley & Sons: Weinheim, Germany, 2005. [Google Scholar]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEAs): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Dalal, S.; Kapoor, M.; Gupta, M.N. Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J. Mol. Catal. B Enzym. 2007, 44, 128–132. [Google Scholar] [CrossRef]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-cleas): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef]
- Xu, M.-Q.; Wang, S.-S.; Li, L.-N.; Gao, J.; Zhang, Y.-W. Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 2018, 8, 460. [Google Scholar] [CrossRef]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Amaral-Fonseca, M.; Kopp, W.; Giordano, R.; Fernández-Lafuente, R.; Tardioli, P. Preparation of magnetic cross-linked amyloglucosidase aggregates: Solving some activity problems. Catalysts 2018, 8, 496. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Ruiz, M.; Cruz, J.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochem. 2012, 47, 2373–2378. [Google Scholar] [CrossRef]
- Guimarães, J.; Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P. Evaluation of strategies to produce highly porous cross-linked aggregates of porcine pancreas lipase with magnetic properties. Molecules 2018, 23, 2993. [Google Scholar] [CrossRef]
- Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose production using starch from cassava bagasse catalyzed by cross-linked β-amylase aggregates. Catalysts 2018, 8, 170. [Google Scholar] [CrossRef]
- Torres, M.P.G.; Foresti, M.L.; Ferreira, M.L. Effect of different parameters on the hydrolytic activity of cross-linked enzyme aggregates (CLEAs) of lipase from Thermomyces lanuginosa. Biochem. Eng. J. 2013, 72, 18–23. [Google Scholar] [CrossRef]
- Torres, M.P.G.; Foresti, M.L.; Ferreira, M.L. CLEAs of Candida antarctica lipase B (CALB) with a bovine serum albumin (BSA) cofeeder core: Study of their catalytic activity. Biochem. Eng. J. 2014, 90, 36–43. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Beltrame, M.B.; Ulrich, L.G.; Giordano, R.; Ribeiro, M.P.; Tardioli, P.W. Combined CLEAs of invertase and soy protein for economically feasible conversion of sucrose in a fed-batch reactor. Food Bioprod. Process. 2018, 110, 145–157. [Google Scholar] [CrossRef]
- Ramos, M.D.; Miranda, L.P.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. 1,3-Regiospecific ethanolysis of soybean oil catalyzed by crosslinked porcine pancreas lipase aggregates. Biotechnol. Prog. 2018, 34, 910–920. [Google Scholar] [CrossRef]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzym. Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef]
- Hecht, H.J.; Schomburg, D.; Kalisz, H.; Schmid, R.D. The 3D structure of glucose oxidase from Aspergillus niger. Implications for the use of GOD as a biosensor enzyme. Biosens. Bioelectron. 1993, 8, 197–203. [Google Scholar] [CrossRef]
- Sund, H.; Weber, K.; Mölbert, E. Dissociation of beef liver catalase in its subunits. Eur. J. Biochem. 1967, 1, 400–410. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J. Biotechnol. 2007, 132, 23–31. [Google Scholar] [CrossRef]
- Wilson, L.; Betancor, L.; Fernández-Lorente, G.; Fuentes, M.; Hidalgo, A.; Guisán, J.M.; Pessela, B.C.C.; Fernández-Lafuente, R. Cross-linked aggregates of multimeric enzymes: A simple and efficient methodology to stabilize their quaternary structure. Biomacromolecules 2004, 5, 814–817. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Abián, O.; Pessela, B.C.C.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of penicillin G acylase and polyionic polymers: An easy methodology to prepare enzyme biocatalysts stable in organic media. Biomacromolecules 2004, 5, 852–857. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef]
- Ayhan, F.; Dogaç, Y.I.; Ayhan, H. Cross-linked glucose oxidase aggregates: Synthesis and characterization. Hacet. J. Biol. Chem. 2011, 39, 241–251. [Google Scholar]
- Cui, J.D.; Liu, R.L.; Li, L.B. A facile technique to prepare cross-linked enzyme aggregates of bovine pancreatic lipase using bovine serum albumin as an additive. Korean J. Chem. Eng. 2016, 33, 610–615. [Google Scholar] [CrossRef]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Hestekin, J.A.; Lin, Y.P.; Frank, J.R.; Snyder, S.W.; Martin, E.J.S. Electrochemical enhancement of glucose oxidase kinetics: Gluconic acid production with anion exchange membrane reactor. J. Appl. Electrochem. 2002, 32, 1049–1052. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process. Synth. 2014, 3, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.L.; Cox, M.M. Princípios de Bioquímica de Lehninger, 5th ed.; Artmed: Porto Alegre, Brazil, 2011. [Google Scholar]
- Cerri, M.O.; Baldacin, J.C.; Cruz, A.J.G.; Hokka, C.O.; Badino, A.C. Prediction of mean bubble size in pneumatic reactors. Biochem. Eng. J. 2010, 53, 12–17. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]




| Precipitant | GOD Activity in the Re-Dissolved Precipitate (%) | CAT Activity in the Re-Dissolved Precipitate (%) |
|---|---|---|
| Dimethoxyethane | 98.3 ± 9.3 | 87.6 ± 8.6 |
| Tert-butyl alcohol | 80.6 ± 7.1 | 77.4 ± 6.9 |
| Saturated ammonium sulfate solution | 5.6 ± 0.9 | 90.2 ± 9.1 |
| Immobilized Catalyst | Bioreactor | Reaction Conditions | Glucose Conversion (%) | GA Productivity (g L−1 h−1) | Reference |
|---|---|---|---|---|---|
| GOD/CAT self-assembled (GOD and CAT matrix via supramolecular recognition of β-cyclodextrin (grafted GOD) and adamantine (grafted CAT)) | Pneumatic with addition of 800 mL min−1 air | 35 °C, pH 5.8, 100–300 g L−1 glucose, 10–26 h | 100 | 5.78–5.88 | [22] |
| GOD/CAT crosslinked with genipin | Batch bioreactor | 35 °C, pH 7.0, 150 g L−1 glucose, 15 h | 100 | 10.89 | [20] |
| GOD/CAT co-immobilized on glutaraldehyde modified porous amino resin | Pneumatic with at 12 L min−1 air flow rate | 35 °C, pH 6.0, 100 g L−1 glucose, 12 h | 95 | 8.62 | [29] |
| GOD and fine palladium particles entrapped within calcium alginate beads | Three different airlift reactors | 30 °C, pH 6.0, 3.4 g L−1 glucose, 2.0–4.5 h | 100 | ~0.8–1.6 | [7] |
| GOD immobilized on anion-exchange membrane | Membrane | pH 4.5–5.5, 1.0 g L−1 glucose, 6.7 h | 90 | 0.13 | [67] |
| GOD/CAT immobilized on an acrylonitrile copolymer membrane adjacent to anion-exchange membrane | Membrane | 26 °C, pH 6.0, 1 g L−1 glucose, 6.0 h | 98 | 0.18 | [5] |
| GOD/CAT combi-CLEA, 1st batch | Bubble column reactor | 40 °C, pH 6.0, 26 g L−1 glucose, 5 h | 96 | 5.42 | Present work |
| GOD/CAT combi-CLEA, after 11th batch (1st batch plus 10 reuses) | Bubble column reactor | 40 °C, pH 6.0, 26 g L−1 glucose, 5 h | 45 | 2.56 | Present work |
| GOD/CAT | Km,app (mM) | kcat,app (h−1) 1 | kcat,app/Km,app | R2 |
|---|---|---|---|---|
| Soluble | 51.1 ± 13.4 | (1.16·± 0.12) × 104 | 227.0 | 0.95 |
| Combi-CLEAs | 65.8 ± 18.5 | (9.82 ± 1.25) × 103 | 149.2 | 0.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafra, A.C.O.; Ulrich, L.G.; Kornecki, J.F.; Fernandez-Lafuente, R.; Tardioli, P.W.; Ribeiro, M.P.d.A. Combi-CLEAs of Glucose Oxidase and Catalase for Conversion of Glucose to Gluconic Acid Eliminating the Hydrogen Peroxide to Maintain Enzyme Activity in a Bubble Column Reactor. Catalysts 2019, 9, 657. https://doi.org/10.3390/catal9080657
Mafra ACO, Ulrich LG, Kornecki JF, Fernandez-Lafuente R, Tardioli PW, Ribeiro MPdA. Combi-CLEAs of Glucose Oxidase and Catalase for Conversion of Glucose to Gluconic Acid Eliminating the Hydrogen Peroxide to Maintain Enzyme Activity in a Bubble Column Reactor. Catalysts. 2019; 9(8):657. https://doi.org/10.3390/catal9080657
Chicago/Turabian StyleMafra, Agnes Cristina Oliveira, Letícia Gazzotto Ulrich, Jakub F. Kornecki, Roberto Fernandez-Lafuente, Paulo Waldir Tardioli, and Marcelo Perencin de Arruda Ribeiro. 2019. "Combi-CLEAs of Glucose Oxidase and Catalase for Conversion of Glucose to Gluconic Acid Eliminating the Hydrogen Peroxide to Maintain Enzyme Activity in a Bubble Column Reactor" Catalysts 9, no. 8: 657. https://doi.org/10.3390/catal9080657
APA StyleMafra, A. C. O., Ulrich, L. G., Kornecki, J. F., Fernandez-Lafuente, R., Tardioli, P. W., & Ribeiro, M. P. d. A. (2019). Combi-CLEAs of Glucose Oxidase and Catalase for Conversion of Glucose to Gluconic Acid Eliminating the Hydrogen Peroxide to Maintain Enzyme Activity in a Bubble Column Reactor. Catalysts, 9(8), 657. https://doi.org/10.3390/catal9080657