Synthesis of ZnO Hollow Microspheres and Analysis of Their Gas Sensing Properties for n-Butanol
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of ZnO Hollow Microspheres
2.3. Characterization of ZnO Hollow Microspheres
2.4. Fabrication and Gas Sensing Test of ZnO Based Gas Sensors
3. Results and Discussion
3.1. Morphology and Microstructure Analysis of ZnO Hollow Microspheres
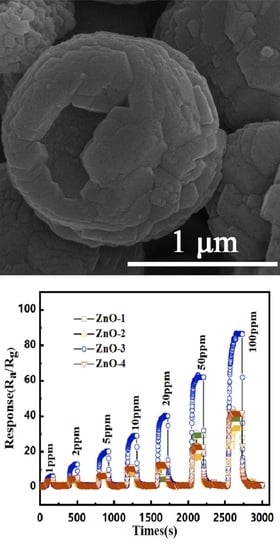
3.2. Gas Sensing Properties of ZnO Hollow Microspheres
3.3. Gas Sensing Mechanism of the ZnO Hollow Microspheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saboor, F.H.; Khodadadi, A.A.; Mortazavi, Y.; Asgari, M. Microemulsion synthesized silica/ZnO stable core/shell sensors highly selective to ethanol with minimum sensitivity to humidity. Sens. Actuator B Chem. 2017, 238, 1070–1083. [Google Scholar] [CrossRef]
- Hang, T.; Wu, J.; Xiao, S.; Li, B.; Li, H.; Yang, C.; Yang, C.; Hu, N.; Xu, Y.; Zhang, Y.; et al. Anti-biofouling NH3 gas sensor based on reentrant thorny ZnO/graphene hybrid nanowalls. Microsyst. Nanoeng. 2020, 6, 41. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, P. Optoelectronic performances on different structures of Al-doped ZnO. J. Am. Ceram. Soc. 2018, 101, 5615–5626. [Google Scholar] [CrossRef]
- Li, C.; Han, C.; Zhang, Y.; Zang, Z.; Wang, M.; Tang, X.; Du, J. Enhanced photoresponse of self-powered perovskite photodetector based on ZnO nanoparticles decorated CsPbBr3 films. Sol. Energy Mater. Sol. Cells 2017, 172, 341–346. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Zheng, K.; She, J.; Deng, S.; Xu, N.; Chen, J. Fabrication of large-area ZnO nanowire field emitter arrays by thermal oxidation for high-current application. Appl. Surf. Sci. 2019, 484, 966–974. [Google Scholar] [CrossRef]
- Kuo, T.-J.; Lin, C.-N.; Kuo, C.-L.; Huang, M.H. Growth of ultralong ZnO nanowires on silicon substrates by vapor transport and their use as recyclable photocatalysts. Chem. Mater. 2007, 19, 5143–5147. [Google Scholar] [CrossRef]
- Saito, B.N.; Haneda, H.; Sekiguchi, T.; Ohashi, N.; Sakaguchi, I.; Koumoto, K. Low-Temperature Fabrication of Light-Emitting Zinc Oxide Micropatterns Using Self-Assembled Monolayers. Adv. Mater. 2002, 14, 418–421. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Yu, Q.; Zhao, L.; Sun, P.; Wang, T.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuator B Chem. 2019, 281, 415–423. [Google Scholar] [CrossRef]
- Lynall, D.; Tseng, A.C.; Nair, S.V.; Savelyev, I.G.; Blumin, M.; Wang, S.; Wang, Z.M.; Ruda, H.E. Nonlinear Chemical Sensitivity Enhancement of Nanowires in the Ultralow Concentration Regime. ACS Nano 2020, 14, 964–973. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, S.K.; Oh, N.C.; Lee, H.D.; Lee, S.M.; Park, J.; Kim, H. Improved Sensitivity in Schottky Contacted Two-Dimensional MoS2 Gas Sensor. ACS Appl. Mat. Interfaces 2019, 11, 38902–38909. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, L.; Li, G.-D.; Zou, X. Well-Tuned Surface Oxygen Chemistry of Cation Off-Stoichiometric Spinel Oxides for Highly Selective and Sensitive Formaldehyde Detection. Chem. Mater. 2018, 30, 2018–2027. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Chen, X.; Lu, G.; Song, X.; Tian, J.; Cui, H.; Zhang, G.; Gao, K. SnO2 core-shell hollow microspheres co-modification with Au and NiO nanoparticles for acetone gas sensing. Powder Technol. 2020, 364, 159–166. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, S.; Liu, J.; He, C.; Lu, G.; Liu, W. Synthesis of Mesoporous SnO2 Spheres and Application in Gas Sensors. Eur. J. Inorg. Chem. 2014, 2014, 863–869. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Zheng, L.; Zhao, Y.; Zheng, W.; Liu, X.; Zhang, J. Hierarchical NiCo2O4 microspheres assembled by nanorods with p-type response for detection of triethylamine. Chin. Chem. Lett. 2020, 31, 2077–2082. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Xue, Y.; Wang, X.; Cui, H.; Tian, J. Homophase structure for promoting electron transfer in gas-sensing. Sens. Actuator B Chem. 2019, 298, 126940. [Google Scholar] [CrossRef]
- Chang, J.; Ahmad, M.Z.; Wlodarski, W.; Waclawik, E.R. Self-assembled 3D ZnO porous structures with exposed reactive {0001} facets and their enhanced gas sensitivity. Sensors 2013, 13, 8445–8460. [Google Scholar] [CrossRef]
- Li, Z.; Pan, W.; Zhang, D.; Zhan, J. Morphology-dependent gas-sensing properties of ZnO nanostructures for chlorophenol. Chem. Asian J. 2010, 5, 1854–1859. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Z.; Qin, N.; Cheng, Z.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuator B Chem. 2017, 242, 148–157. [Google Scholar] [CrossRef]
- Liu, F.; Huang, G.; Wang, X.; Xie, X.; Xu, G.; Lu, G.; He, X.; Tian, J.; Cui, H. High response and selectivity of single crystalline ZnO nanorods modified by In2O3 nanoparticles for n-butanol gas sensing. Sens. Actuator B Chem. 2018, 277, 144–151. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Yue, J.; Jiang, X.; Yu, A. Controllable Synthesis of ZnO Nanoflakes with Exposed (100) for Enhanced Gas Sensing Performance. J. Phys. Chem. C 2013, 117, 13153–13162. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Gu, C.; Zhai, M.; Yu, K.; Yang, M.; Liu, J. Large-scale synthesis of flowerlike ZnO nanostructure by a simple chemical solution route and its gas-sensing property. Sens. Actuator B Chem. 2010, 146, 206–212. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Xing, X.; Li, Y.; Xiao, X.; Wang, Y.; Djerdj, I. A high-performance n-butanol gas sensor based on ZnO nanoparticles synthesized by a low-temperature solvothermal route. RSC Adv. 2015, 5, 54372–54378. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Ma, X.; Mi, R.; Chen, Y.; Ruan, S. The significant improvement for BTX (benzene, toluene and xylene) sensing performance based on Au-decorated hierarchical ZnO porous rose-like architectures. Sens. Actuator B Chem. 2018, 262, 86–94. [Google Scholar] [CrossRef]
- Han, B.; Liu, X.; Xing, X.; Chen, N.; Xiao, X.; Liu, S.; Wang, Y. A high response butanol gas sensor based on ZnO hollow spheres. Sens. Actuator B Chem. 2016, 237, 423–430. [Google Scholar] [CrossRef]
- Guo, W.; Liu, T.; Sun, R.; Chen, Y.; Zeng, W.; Wang, Z. Hollow, porous, and yttrium functionalized ZnO nanospheres with enhanced gas-sensing performances. Sens. Actuator B Chem. 2013, 178, 53–62. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Yang, T.; Zhao, J.; Wang, Z.; Wang, W.; Liu, Y.; Lu, G.; Du, Y. Template-free microwave-assisted synthesis of ZnO hollow microspheres and their application in gas sensing. CrystEngComm 2013, 15, 2949–2955. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Chemiresistive sensors based on core-shell ZnO@TiO2 nanorods designed by atomic layer deposition for n-butanol detection. Sens. Actuator B Chem. 2020, 310, 127846. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, P.; Wang, Y.; Xu, L.; Zhang, B.; Lv, C.; Ma, J.; Zhang, Y. Sb-doped three-dimensional ZnFe2O4 macroporous spheres for N-butanol chemiresistive gas sensors. Sens. Actuator B Chem. 2020, 320, 128384. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, P.; Sun, D.; Sun, Y.; Zhang, G.; Gao, D. Tunable synthesis of uniform ZnO nanospheres and their size-dependent gas sensing performance toward n-butanol. Mater. Lett. 2015, 161, 495–498. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuator B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Saidi, T.; Palmowski, D.; Babicz-Kiewlicz, S.; Welearegay, G.T.; Bari, N.E.; Smulko, J.; Bouchikhi, B. Exhale breath gas sensing using pristine and functionalized WO3 nanowire sensors enhanced by UV-light irradiation. Sens. Actuator B Chem. 2018, 273, 1719–1729. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Qiao, G.; Chen, X.; Wang, X.; Cui, H. Synthesis of ZnO Hollow Microspheres and Analysis of Their Gas Sensing Properties for n-Butanol. Crystals 2020, 10, 1010. https://doi.org/10.3390/cryst10111010
Wang S, Qiao G, Chen X, Wang X, Cui H. Synthesis of ZnO Hollow Microspheres and Analysis of Their Gas Sensing Properties for n-Butanol. Crystals. 2020; 10(11):1010. https://doi.org/10.3390/cryst10111010
Chicago/Turabian StyleWang, Shichao, Gaoqun Qiao, Xiaoyan Chen, Xinzhen Wang, and Hongzhi Cui. 2020. "Synthesis of ZnO Hollow Microspheres and Analysis of Their Gas Sensing Properties for n-Butanol" Crystals 10, no. 11: 1010. https://doi.org/10.3390/cryst10111010
APA StyleWang, S., Qiao, G., Chen, X., Wang, X., & Cui, H. (2020). Synthesis of ZnO Hollow Microspheres and Analysis of Their Gas Sensing Properties for n-Butanol. Crystals, 10(11), 1010. https://doi.org/10.3390/cryst10111010