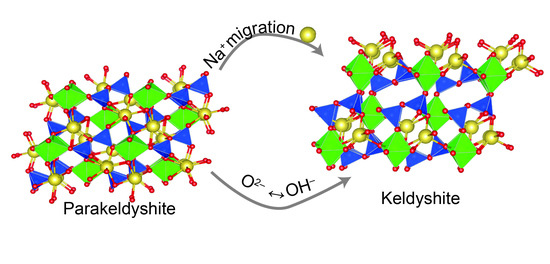
The Na2−nHn[Zr(Si2O7)]∙mH2O Minerals and Related Compounds (n = 0–0.5; m = 0.1): Structure Refinement, Framework Topology, and Possible Na+-Ion Migration Paths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Composition
2.3. Single-Crystal X-ray Diffraction
2.4. Geometrical–Topological Analysis
2.5. Raman Spectroscopy
3. Results
3.1. Single-Crystal X-ray Diffraction
3.2. Topological Analysis
3.3. Raman Spectroscopy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K. Crystal chemistry, properties and synthesis of microporous silicates containing transition elements. Russ. Chem. Rev. 2004, 73, 205–223. [Google Scholar] [CrossRef]
- Ferreira, P.; Ferreira, A.; Rocha, J.; Soares, M.R. Synthesis and Structural Characterization of Zirconium Silicates. Chem. Mater. 2001, 13, 355–363. [Google Scholar] [CrossRef]
- Chukanov, N.V.V.; Pekov, I.V. Heterosilicates with Tetrahedral-Octahedral Frameworks: Mineralogical and Crystal-Chemical Aspects. Rev. Mineral. Geochem. 2005, 57, 105–143. [Google Scholar] [CrossRef]
- Ilyushin, G.D.; Dem’yanets, L.N.; Ilyukhin, V.V.; Belov, N.V. Crystallization analougs of minerals and synthetic phases in the hydrotermal system NaOH–ZrO2–SiO2–H2O. Dokl. Akad. Nauk SSSR 1983, 271, 1133–1136. (In Russian) [Google Scholar]
- Ilyushin, G.D. Crystallization in the Na2CO3‒ZrO2–SiO2–H2O system at 450 °С and 0.1–0.05 GPa. Inorg. Mater. 2002, 38, 1249–1257. (In Russian) [Google Scholar]
- Ilyushin, G.D. Hydrotermal crystallization in the system NaOH–ZrO2–SiO2–H2O at 450 °C: Phase relations Na4Zr2Si5O16 · H2O, Na8ZrSi6O18, Na3HZrSi2O8, Na4Zr2Si3O12. J. Inorg. Chem. 2003, 48, 1002–1011. (In Russian) [Google Scholar]
- Bortun, A.I.; Bortun, L.N.; Clearfield, A. Hydrothermal Synthesis of Sodium Zirconium Silicates and Characterization of Their Properties. Chem. Mater. 1997, 9, 1854–1864. [Google Scholar] [CrossRef]
- Jale, S.R.; Ojo, A.; Fitch, F.R. Synthesis of microporous zirconosilicates containing ZrO6 octahedra and SiO4 tetrahedra. Chem. Commun. 1999, 411–412. [Google Scholar] [CrossRef]
- Poojary, D.M.; Bortun, A.I.; Bortun, L.N.; Clearfield, A. Syntheses and X-ray Powder Structures of K2(ZrSi3O9)·H2O and Its Ion-Exchanged Phases with Na and Cs. Inorg. Chem. 1997, 36, 3072–3079. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.K.; Férey, G.; Loiseau, T. Open-Framework Inorganic Materials. Angew. Chemie Int. Ed. 1999, 38, 3268–3292. [Google Scholar] [CrossRef]
- Gerasimovskii, V.I. Keldyshite—A new mineral. Dokl. Akad. Nauk SSSR 1962, 142, 916–918. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Kazakova, M.E.; Voronkov, A.A. New data on keldyshite, Doklady Akademii Nauk SSSR. Dokl. Akad. Nauk SSSR 1969, 189, 166–168. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Kazakova, M.E.; Vlasova, E.V.; Smolyaninova, N.N. Investigation on minerals of the keldyshite group. Tr. Mineral. Muzeya Fersman Akad. Nauk SSSR 1975, 24, 120–131. (In Russian) [Google Scholar]
- Khomyakov, A.P. Types of regular intergrowths of minerals of the keldyshite group. Geochemistry. Mineral. Intern. Geol. Congr. XXV Sess. Dokl. Owls. Geol. M 1976, 25, 233–240. (In Russian) [Google Scholar]
- Khomyakov, A.P. Parakeldyshite a new mineral. Dokl. Akad. Nauk SSSR 1977, 237, 703–705. (In Russian) [Google Scholar]
- Khomyakov, A.P. Keldyshite group minerals. Priroda 2011, 12, 35–39. (In Russian) [Google Scholar]
- Voronkov, A.A.; Shumyatskaya, N.G.; Pyatenko, Y.A. Crystal structure of a new natural modification of Na2Zr[Si2O7]. J. Struct. Chem. 1970, 11, 866–867. (In Russian) [Google Scholar] [CrossRef]
- Sizova, R.G.; Kuz’min, E.A.; Ilyukhin, V.V. Deciphering the continuous Patterson function of the Na,Zr-diorthosilicate Na2ZrSi2O7 (parakeldyshite) by the vector subsystem method. Konstitutsiya Svoistva Miner. 1975, 9, 10–12. (In Russian) [Google Scholar]
- Khalilov, A.D.; Khomyakov, A.P.; Makhmudov, S.A. Crystal structure of keldyshite Na2[Si2O6OH]. Dokl. Akad. Nauk SSSR 1978, 238, 573–575. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Semenov, E.I.; Es’kova, E.M.; Voronkov, A.A. Kazakovite—A new mineral of the lovozerite group. Zap. RMO 1974, 103, 342–345. (In Russian) [Google Scholar]
- Khomyakov, A.P. New data on mineralogy of lovozerite group. Dokl. Akad. Nauk SSSR 1977, 237, 199–202. (In Russian) [Google Scholar]
- Khomyakov, A.P. Mineralogy of Ultraagpaitic Alkaline Rocks; Nauka: Moscow, Russia, 1990. (In Russian) [Google Scholar]
- Khomyakov, A.P. Typomorphism of minerals of ultraagpaitic pegmatites. In Scientific Foundations and Practical Use of Mineral Typomorphism; Nauka: Moscow, Russia, 1980; pp. 152–157. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Yushkin, N.P. The principle of inheritance in crystallogenesis. Dokl. Akad. Nauk SSSR 1981, 256, 1229–1233. (In Russian) [Google Scholar]
- Khomyakov, A.P. Ultraagpaite rocks of the Khibino-Lovozersky complex as an inexhaustible source minerals with unique properties. In Fersman Scientific Session; KSC Press: Apatity, Russia, 2007; pp. 202–205. (In Russian) [Google Scholar]
- Yushkin, N.P.; Khomyakov, A.P.; Evzikova, N.Z. The Principle of Inheritance in Mineralogenesis; Nauka: Syktyvkar, Russia, 1984. (In Russian) [Google Scholar]
- Pekov, I.V.; Zubkova, N.V.; Pushcharovsky, D.Y.; Kolich, U.; Tillmanns, E. Refined crystal structure of parakeldyshite and genetic crystal chemistry of zirconium minerals with diortho Si2O7 groups. Kristallografiya 2007, 52, 1100–1105. (In Russian) [Google Scholar]
- Nikolova, R.P.; Fujiwara, K.; Nakayama, N.; Kostov-Kytin, V. Crystal structure of a new small-pore zirconosilicate Na2ZrSi2O7·H2O and its relation to stoichiometrically and topologically similar compounds. Solid State Sci. 2009, 11, 382–388. [Google Scholar] [CrossRef]
- Chelishchev, N.F.; Khomyakov, A.P.; Berenshtein, B.G. Author’s certificate AS No. 1096794 dated 02/08/1984 “Method for cleaning gases from sulfur dioxide”. Bul. Discov. Invent. 1984, 21, 1–20. (In Russian) [Google Scholar]
- Ilyushin, G.D.; Dem’yanets, L.N. Crystal structural features of ion transport in new OD-structures: Catapleiite Na2ZrSi3O9 2H2O and hilairite Na2ZrSi3O9 3H2O. Kristallografiya 1988, 33, 383–387. (In Russian) [Google Scholar]
- Zubkova, N.V.; Pekov, I.V.; Turchkova, A.G.; Pushcharovsky, D.Y.; Merlino, S.; Pazero, M.; Chukanov, N.V. Crystal structures of potassium-substituted forms of catapleiite and hilairite. Kristallografiya 2007, 52, 68–72. (In Russian) [Google Scholar]
- Zubkova, N.V.; Ksenofontov, D.A.; Chukanov, N.V.; Pekov, I.V.; Artamonova, A.A.; Koshlyakova, N.N.; Bychkov, A.Y.; Pushcharovsky, D.Y. Crystal Chemistry of the Microporous Zirconosilicate Na6Zr3[Si9O27], a Product of High-Temperature Transformation of Catapleiite, and Its Ag-Exchanged Form. Minerals 2020, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Blatova, O.A.; Ivanovschitz, A.; Dem’yanets, L.N. Migration maps of Li+ cations in oxygen-containing compounds. Solid State Ionics 2008, 179, 2248–2254. [Google Scholar] [CrossRef]
- Agilent Technologies CrysAlis CCD and CrysAlis RED; Oxford Diffr. Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Eremin, R.A.; Kabanova, N.A.; Morkhova, Y.A.; Golov, A.A.; Blatov, V.A. High-throughput search for potential potassium ion conductors: A combination of geometrical-topological and density functional theory approaches. Solid State Ionics 2018, 326, 188–199. [Google Scholar] [CrossRef]
- Fedotov, S.S.; Kabanova, N.A.; Kabanov, A.A.; Blatov, V.A.; Khasanova, N.R.; Antipov, E.V. Crystallochemical tools in the search for cathode materials of rechargeable Na-ion batteries and analysis of their transport properties. Solid State Ionics 2018, 314, 129–140. [Google Scholar] [CrossRef]
- Meutzner, F.; Münchgesang, W.; Kabanova, N.A.; Zschornak, M.; Leisegang, T.; Blatov, V.A.; Meyer, D.C. On the Way to New Possible Na-Ion Conductors: The Voronoi-Dirichlet Approach, Data Mining and Symmetry Considerations in Ternary Na Oxides. Chem. A Eur. J. 2015, 21, 16601–16608. [Google Scholar] [CrossRef]
- Ilyushin, G.D.; Blatov, V.A. Crystal chemistry of zirconosilicates and their analogs: Topological classification of MT frameworks and suprapolyhedral invariants. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 198–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: Recommended terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A. Methods for topological analysis of atomic networks. J. Struct. Chem. 2009, 50, 160–167. [Google Scholar]
- Blatova, O.A.; Golov, A.A.; Blatov, V.A. Natural tilings and free space in zeolites: Models, statistics, correlations, prediction. Z. Krist. Cryst. Mater. 2019, 234, 421–436. [Google Scholar] [CrossRef]
- Chong, S.; Aksenov, S.M.; Dal Bo, F.; Perry, S.N.; Dimakopoulou, F.; Burns, P.C. Framework Polymorphism and Modular Crystal Structures of Uranyl Vanadates of Divalent Cations: Synthesis and Characterization of M(UO2)(V2O7) (M = Ca,Sr) and Sr3(UO2)(V2O7)2. Z. Anorg. Allg. Chem. 2019, 645, 981–987. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K.; Hixon, A.E. Crystal structure and topological features of manganonaujakasite, a mineral with microporous heteropolyhedral framework related to AlPO-25 (ATV). Microporous Mesoporous Mater. 2019, 279, 128–132. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Mackley, S.A.; Deyneko, D.V.; Taroev, V.K.; Tauson, V.L.; Rastsvetaeva, R.K.; Burns, P.C. Crystal chemistry of compounds with lanthanide based microporous heteropolyhedral frameworks: Synthesis, crystal structures, and luminescence properties of novel potassium cerium and erbium silicates. Microporous Mesoporous Mater. 2019, 284, 25–35. [Google Scholar] [CrossRef]
- Blatov, V.A.; Delgado-Friedrichs, O.; O’Keeffe, M.; Proserpio, D.M. Three-periodic nets and tilings: Natural tilings for nets. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 63, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Baur, W.H. The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1974, 30, 1195–1215. [Google Scholar] [CrossRef]
- Chernov, A.N.; Maksimov, V.A.; Ilyukhin, V.V.; Belov, N.V. Crystalline structure of monoclinic modification of K,Zr-diorthosilicate (K2ZrSi2O7). Sov. Phys. Dokl. 1971, 15, 711–713. (In Russian) [Google Scholar]
- Pertierra, P.; Salvado, M.A.; Garcia-Granda, S.; Trabajo, C.; Garcia, J.R.; Bortun, A.I.; Clearfield, A. Synthesis, Characterization, and X-Ray Powder Structure of K2ZrGe2O7. J. Solid State Chem. 1999, 148, 41–49. [Google Scholar] [CrossRef]
- Faggiani, R.; Crispin, C. Crystal structure of CaK, AsrO, and GdK2P,0. Can. J. Chem. 1976, 54, 3319–3324. [Google Scholar] [CrossRef] [Green Version]
- Leclaire, A.; Benmoussa, A.; Borel, M.M.; Grandin, A.; Raveau, B. Two forms of sodium titanium(III) diphosphate: α-NaTiP2O7 closely related to β-cristobalite and β-NaTiP2O7 isotypic with NaFeP2O7. J. Solid State Chem. 1988, 77, 299–305. [Google Scholar] [CrossRef]
- Fleet, M.E.; Henderson, G.S. Sodium trisilicate: A new high-pressure silicate structure. Phys. Chem. Miner. 1995, 22, 383–386. [Google Scholar] [CrossRef]
- Hamady, A.; Zid, M.F.; Jouini, T. Structure cristalline de KYP2O7. J. Solid State Chem. 1994, 113, 120–124. [Google Scholar] [CrossRef]
- Muller-Buschbaum, H.K.; Rutter, I. A New Structure on Barium Lanthanoid Aluminates: Ba6Dy2Al4O15. Z. Anorg. Allg. Chem. 1989, 573, 89–94. [Google Scholar]
- Poojary, D.M.; Borade, R.B.; Campbell, F.L.; Clearfield, A. Crystal Structure of Silicon Pyrophosphate (Form I) from Powder Diffraction Data. J. Solid State Chem. 1994, 112, 106–112. [Google Scholar] [CrossRef]
- Newnham, R.E.; Redman, M.J.; Santoro, R.P. Crystal Structure of Yttrium and Other Rare-Earth Borates. J. Am. Ceram. Soc. 1963, 46, 253–256. [Google Scholar] [CrossRef]
- Skshat, S.M.; Simonov, V.; Belov, N.V. Crystal structure of the synthetic sodium-scandium silicate Na3Sc(Si2O7). Dokl. Akad. Nauk SSSR 1969, 184, 337–340. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Walter de Gruyter GmbH: Berlin, Germany, 2015; pp. 1–30. [Google Scholar] [CrossRef]
- Sitarz, M.; Mozgawa, W.; Handke, M. Vibrational spectra of complex ring silicate anions—Method of recognition. J. Mol. Struct. 1997, 404, 193–197. [Google Scholar] [CrossRef]
- Frost, R.L.; Bouzaid, J.M.; Martens, W.N.; Reddy, B.J. Raman spectroscopy of the borosilicate mineral ferroaxinite. J. Raman Spectrosc. 2007, 38, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, R.J.; Yarger, J.L.; McMillan, P.F.; Ping, Y.; Cong, X. Raman spectroscopy of C-S-H, tobermorite, and jennite. Adv. Cem. Based Mater. 1997, 5, 93–99. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P. A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Konopleva, N.G.; Panikorovskii, T.L.; Bazai, A.; Mikhailova, J.A.; Bocharov, V.N.; Ivanyuk, G.Y.; Krivovichev, S.V. Batagayite, CaZn2(Zn,Cu)6(PO4)4(PO3OH)3·12H2O, a new phosphate mineral from Këster tin deposit (Yakutia, Russia): Occurrence and crystal structure. Mineral. Petrol. 2018, 112, 591–601. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Mineralogy of Hyperagpaitic Alkaline Rocks; Clarendon Press: Oxford, UK, 1995. [Google Scholar]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Zolotarev, A.A.J.; Krivovichev, S.V.; Panikorovskii, T.L.; Gurzhiy, V.V.; Bocharov, V.N.; Rassomakhin, M.A. Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]











| Parameter | Data |
|---|---|
| Temperature/K | 293(2) |
| Crystal system | triclinic |
| Space group | P |
| a/Å | 5.4243(6) |
| b/Å | 6.5923(5) |
| c/Å | 8.8083(6) |
| α/° | 71.309(7) |
| β/° | 87.162(8) |
| γ/° | 85.497(8) |
| Volume/Å3 | 297.34(5) |
| Z | 2 |
| ρcalc/g cm–3 | 3.411 |
| μ/mm-1 | 2.388 |
| F(000) | 292.0 |
| Crystal size/mm3 | 0.17 × 0.12 × 0.11 |
| Radiation | Mo Kα (λ = 0.71073) |
| 2θ range for data collection/° | 6.538 to 54.986 |
| Index ranges | −5 ≤ h ≤ 7, −8 ≤ k ≤ 8, −11 ≤ l ≤ 10 |
| Reflections collected | 2296 |
| Independent reflections | 1364 [Rint = 0.0221, Rσ = 0.0362] |
| Data/restraints/parameters | 1364/0/109 |
| Goodness-of-fit on F2 | 1.120 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0237, wR2 = 0.0602 |
| Final R indexes [all data] | R1 = 0.0256, wR2 = 0.0616 |
| Largest diff. peak/hole/e Å−3 | 0.79/−0.69 |
| Formula | Space Group | Topology | ICSD Code | Ref. |
|---|---|---|---|---|
| NaH[Zr(Si2O7)] keldyshite | P | fsh | 20186 | [47] |
| Na2[Zr(Si2O7)] parakeldyshite | P | fsh | – | This work |
| K2[Zr(Si2O7)] khibinskite | P21/b | fsh | 20100 | [49] |
| K2[Zr(Ge2O7)] | C2/c | fsh | 88843 | [50] |
| K2[Cd(P2O7)] | C2/c | fsh | 12117 | [51] |
| Na[Ti(P2O7)] | P21/c | fsh | 202751 | [52] |
| Na2[SiVI(SiIV2O7) | C2/c | fsh | 81134 | [53] |
| Na2[Zr(Si2O7)]∙H2O | C2/c | xat | 419420 | [28] |
| K[Y(P2O7)] | Cmcm | xat | 75171 | [54] |
| Ba6Dy2Al4O15 | Cmcm | xat | 85071 | [55] |
| Si(P2O7) | P63 | xat | 75116 | [56] |
| Tm(BO3) | Pc2 | xat | 27942 | [57] |
| Na3[Sc(Si2O7)] | Pbnm | xat | 20120 | [58] |
| Compound | Sp. Gr., Z | Unit Cell Parameters | V, Å3 | Citation | ||
|---|---|---|---|---|---|---|
| a, Å, α, ° | b, Å, β ° | c, Å, γ, ° | ||||
| Keldyshite NaH[Zr(Si2O7)] | P, 2 | 9.01 | 5.34 | 6.96 | 300.39 | [19] |
| 92.1 | 116.1 | 88.1 | ||||
| Parakeldyshite Na2[Zr(Si2O7)] | P, 2 | 8.8083 | 5.4243 | 6.5923 | 297.34 | Current work |
| 87.162 | 85.497 | 71.309 | ||||
| Na2[Zr(Si2O7)]∙H2O | C2/c, 4 | 10.422 | 8.247 | 9.205 | 672.2 | [27] |
| 90 | 116.55 | 90 | ||||
| Compound | Number of Nodes in the Ring | Radius of Ring, Å | Compound | Number of Nodes in the Ring | Radius of Ring, Å |
|---|---|---|---|---|---|
| Parakeldyshite Na2ZrSi2O7 | 6 | 2.12 | Keldyshite NaZr(Si2O6OH) | 6 | 2.09 |
| 6 | 2.06 | 6 | 2.00 | ||
| 6 | 1.75 | 6 | 2.05 | ||
| 6 | 2.14 | 6 | 2.12 | ||
| 6 | 2.06 | 6 | 2.10 | ||
| 4 | 1.53 | 4 | 1.77 | ||
| 4 | 1.54 | 4 | 1.37 | ||
| 4 | 1.80 | 4 | 1.65 | ||
| 4 | 1.66 | 4 | 1.84 | ||
| 4 | 1.64 | 4 | 1.17 | ||
| Na2ZrSi2O7∙H2O | 6 | 2.36 | 4 | 1.77 | |
| 6 | 2.37 | 4 | 1.78 | ||
| 4 | 1.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabanova, N.A.; Panikorovskii, T.L.; Shilovskikh, V.V.; Vlasenko, N.S.; Yakovenchuk, V.N.; Aksenov, S.M.; Bocharov, V.N.; Krivovichev, S.V. The Na2−nHn[Zr(Si2O7)]∙mH2O Minerals and Related Compounds (n = 0–0.5; m = 0.1): Structure Refinement, Framework Topology, and Possible Na+-Ion Migration Paths. Crystals 2020, 10, 1016. https://doi.org/10.3390/cryst10111016
Kabanova NA, Panikorovskii TL, Shilovskikh VV, Vlasenko NS, Yakovenchuk VN, Aksenov SM, Bocharov VN, Krivovichev SV. The Na2−nHn[Zr(Si2O7)]∙mH2O Minerals and Related Compounds (n = 0–0.5; m = 0.1): Structure Refinement, Framework Topology, and Possible Na+-Ion Migration Paths. Crystals. 2020; 10(11):1016. https://doi.org/10.3390/cryst10111016
Chicago/Turabian StyleKabanova, Natalya A., Taras L. Panikorovskii, Vladimir V. Shilovskikh, Natalya S. Vlasenko, Victor N. Yakovenchuk, Sergey M. Aksenov, Vladimir N. Bocharov, and Sergey V. Krivovichev. 2020. "The Na2−nHn[Zr(Si2O7)]∙mH2O Minerals and Related Compounds (n = 0–0.5; m = 0.1): Structure Refinement, Framework Topology, and Possible Na+-Ion Migration Paths" Crystals 10, no. 11: 1016. https://doi.org/10.3390/cryst10111016
APA StyleKabanova, N. A., Panikorovskii, T. L., Shilovskikh, V. V., Vlasenko, N. S., Yakovenchuk, V. N., Aksenov, S. M., Bocharov, V. N., & Krivovichev, S. V. (2020). The Na2−nHn[Zr(Si2O7)]∙mH2O Minerals and Related Compounds (n = 0–0.5; m = 0.1): Structure Refinement, Framework Topology, and Possible Na+-Ion Migration Paths. Crystals, 10(11), 1016. https://doi.org/10.3390/cryst10111016