Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Complexes and Crystallization
2.2. Materials and Methods
3. Results and Discussion
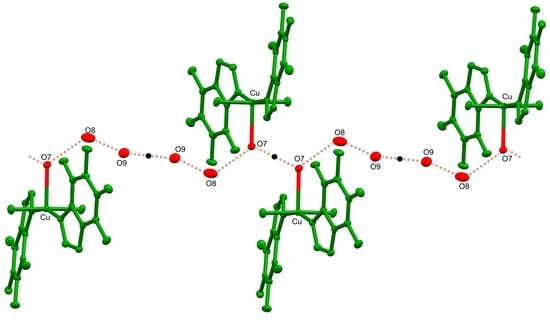
3.1. Crystal Structure
Crystal Packing Analysis
3.2. Infrared Spectra
3.3. TG-DTG-DTA Measurements
3.4. Antioxidant and Antimicrobial Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilbert, R.M. Caffeine Consumption. Prog. Clin. Biol. Res. 1984, 158, 185–213. [Google Scholar] [PubMed]
- Bujdosova, Z.; Gyoryova, K.; Ruzicka, A.; Melnik, M.; Koman, M. Crystal structures of two aromatiic zinc(II) caroboxylates: [Zn(4-chlorosalicylato)2(H2O)4]·2theophylline·(H2O)2 and unique [Zn(5-chlorosalicylato)2(isonicotinamine)2(H2O)]. J. Chem. Crystallogr. 2011, 41, 1077–1084. [Google Scholar] [CrossRef]
- Biradha, K.; Samai, S.; Maity, A.C.; Goswami, S. Supramolecular assembly of protonated xanthine alkaloids in their perchlorate salts. Cryst. Growth Des. 2009, 10, 937–942. [Google Scholar] [CrossRef]
- Fredholm, B.B. Methylxanthines; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Petrucci, R.; Chiarotto, I.; Mattiello, L.; Passeri, D.; Rossi, R.; Zollo, G.; Feroci, M. Graphene Oxide: A smart (starting) material for natural methylxanthines adsorption and detection. Molecules 2019, 24, 4247. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Crit. Rev. Food Sci. Nutr. 2019, 59, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.; Zollo, G.; Curulli, A.; Marrosu, G. A new insight into the oxidative mechanism of caffeine and related methylxanthines in aprotic medium: May caffeine be really considered as an antioxidant? Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [Green Version]
- Kossel, A. Über eine neue Base aus dem Pflanzenreich. Ber. Dtsch. Chem. Ges. 1888, 21, 2164–2167. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.; Ach, L. Synthese des Caffeins. Ber. Dtsch. Chem. Ges. 1985, 28, 3135–3139. [Google Scholar] [CrossRef]
- Sharron, H.F.; Konjeti, R.S.; Hengming, K.; Jackie, D.C. Inhibition of Cyclic Nucleotide Phosphodiesterases by Methylxanthines. In Methylxanthines, Handbook of Experimental Pharmacology; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 93–134. [Google Scholar]
- Santos, C.I.A.V.; Ramos, M.L.; Justino, L.L.G.; Burrows, H.D.; Valente, A.J.M.; Esteso, M.A.; Leaist, D.G.; Ribeiro, A.F.C. Effect of pH in the structure and mass transport by diffusion of theophylline. J. Chem. Thermodyn. 2017, 110, 162–170. [Google Scholar] [CrossRef]
- Barnes, P.J. Pulmonary Perspectives. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, R. Neurobiología de las metilxantinas. Trastor. Adict. 2008, 10, 201–207. [Google Scholar] [CrossRef]
- Peck, C.C.; Nichols, A.I.; Baker, J.; Lenert, L.L.; Ezra, D. Clinical pharmacodynamics of theophylline. J. Allergy Clin. Immunol. 1985, 76, 292–297. [Google Scholar] [CrossRef]
- Ashihara, H.; Crozier, A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 1999; Volume 30, pp. 117–205. [Google Scholar]
- Nishijo, J.; Yonetani, I. The interaction of theophylline with benzylamine in aqueous solution. Chem. Pharm. Bull. 1982, 30, 4507–4511. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.I.A.V.; Ribeiro, A.C.F.; Esteso, M.A. Drug delivery systems: Study of inclusion complex formation between methylxanthines and cyclodextrins and their thermodynamic and transport properties. Biomolecules 2019, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index, 14th ed.; Merck: Rahway, NJ, USA, 2006. [Google Scholar]
- Cavalieri, L.F.; Fox, J.J.; Stone, A.; Chang, N. On the nature of xantine and substituted xanthenes in solution. J. Am. Chem. Soc. 1954, 76, 1119–1122. [Google Scholar] [CrossRef]
- Desoize, B. Metals and metal compounds in cancer treatment. Anticancer Res. 2004, 24, 1529–1544. [Google Scholar]
- Shaw, C.F., III. Gold-based medicinal agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Graf, N.; Lippard, S.J. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Liang, X. Metal-mediated targeting in the body. Chem. Biol. Drug Des. 2013, 81, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Glowniak, I.; Wolf, W.M. Synthesis, characterization, crystal structure and biological activity of metal(II) complexes with theophylline. J. Saudi Chem. Soc. 2019, 23, 346–354. [Google Scholar] [CrossRef]
- El Hamdani, H.; El Amane, M.; Duhayon, C. Crystal structure of tetraaquabis (1,3-dimethyl-2,6-dioxo-7H- purin-7-ido-κ N7)cobalt(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1302–1304. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.S.; Monohar, H. Synthesis and X-ray crystal structure of a CuII-theophylline complex: [Cu(theo) (H2O)]·2H2O. Polyhedron 1994, 13, 307–312. [Google Scholar] [CrossRef]
- Wang, H.; Hu, T.; Wen, R.; Wang, Q.; Bu, X. In vitro controlled release of theophylline from metal-drug complexes. J. Mater. Chem. B 2013, 1, 3879–3882. [Google Scholar] [CrossRef]
- Hao, X.M.; Zhao, S.; Wang, H.; Wu, Y.B.; Yang, D.; Zhang, X.F.; Xu, Z.L. In vitro release of theophylline and cytotoxicity of two new metal–drug complexes. Polyhedron 2018, 142, 38–42. [Google Scholar] [CrossRef]
- Forizs, E.; Debreczeni, A.; Patrut, A. Synthesis, structure and DFT calculations on complexes of palladium (II) with theophylline. Rev. Roum. 2010, 55, 697–704. [Google Scholar]
- Caldwell, K.; Deacon, G.B.; Gatehouse, B.M.; Lee, S.C.; Canty, A.J. Organomercury medicinal chemistry. Synthesis and structure of a (β-methoxyethyl)mercury(II) derivative of N(7)-deprotonated theophylline, [Hg(C3H7O)(C7H7N4O2)]. Acta Cryst. C 1984, 40, 1533–1536. [Google Scholar] [CrossRef]
- Aoki, K.; Yamazaki, H. Interaction of tetrakis(-µ-carboxylato)dirhodiurn(II), an antitumour agent, with nucleic acid bases. X-ray crystal structures of [Rh2(acetato)4(theophylline)2] and [Rh2(acetato)4(caffeine)2]. J. Chem. Soc. Chem. Commun. 1980, 926, 186–188. [Google Scholar] [CrossRef]
- Griffith, E.H.; Amma, E.L. Reaction of PtCl42− with theophylline: X-ray crystal structures of bis(theophyllinum) tetrachloroplatine(II) and theophyllinum trichlorotheophyllineplatinate(II). Inorg. Chem. 1979, 7, 322–324. [Google Scholar]
- Feng, J.; Du, X.; Liu, H.; Sui, X.; Zhang, C.; Tang, Y.; Zhang, J. Manganeseese-mefenamic acid complexes exhibit high lipoxygenase inhibitory activity. Dalton Trans. 2014, 43, 10930–10939. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction; CrysAlisPro 1.171.39.33c; Agilent Technologies UK Ltd.: Yarnton, UK, 2017.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- HüBschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; Mccabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Żesławska, E.; Korona-Głowniak, I.; Szczesio, M.; Olczak, A.; Żylewska, A.; Tejchman, W.; Malm, A. Structural analysis and antimicrobial activity of 2[1H]-pyrimidinethione/selenone derivatives. J. Mol. Struct. 2017, 1142, 261–266. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.; Shimoni, L.; Chang, N. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]











| 1 | |
|---|---|
| Empirical formula | C14H24CuN8O9 |
| Formula weight | 511.95 |
| T (K) | 100.0 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 12.3749(2) |
| b (Å) | 11.9281(3) |
| c (Å) | 13.7162(2) |
| α (°) | 90 |
| β (°) | 98.133(10) |
| γ (°) | 90 |
| V (Å3) | 2004.27(7) |
| Z | 4 |
| ρcalc (g/cm3) | 1.697 |
| F(000) | 1060 |
| Radiation | CuKα (λ = 1.54184) |
| θ range (°) | 4.506–70.052 |
| Reflections collected | 43730 |
| Independent reflections | 3811 |
| Goodness-of-fit (GOF) | 1.061 |
| R [I >= 2σ (I)] | 0.0295 |
| wR2 [I >= 2σ (I)] | 0.0810 |
| D-H⋯A | D-H | H⋯A | D⋯A | D-H⋯A |
|---|---|---|---|---|
| O5-H5A⋯O2 a | 0.77(3) | 1.94(3) | 2.701(2) | 175(3) |
| O5-H5B⋯O3 b | 0.81(2) | 1.94(3) | 2.722(2) | 162(2) |
| O6-H6A⋯N3 c | 0.73(3) | 2.00(3) | 2.721(2) | 174(3) |
| O6-H6B⋯N7 d | 0.77(2) | 1.99(2) | 2.756(2) | 179(3) |
| O7-H7A⋯O8 | 0.80(4) | 1.95(4) | 2.748(2) | 170(5) |
| O7-H7B⋯O7 e | 0.81(4) | 1.94(4) | 2.749(2) | 175(5) |
| O7-H7C⋯O1 | 0.82(2) | 1.87(2) | 2.686(2) | 172(2) |
| O8-H8A⋯O3 f | 0.82(3) | 2.14(3) | 2.956(2) | 173(5) |
| O8-H8B⋯O9 | 0.83(2) | 1.89(4) | 2.712(3) | 170(2) |
| O8-H8C⋯O7 | 0.78(4) | 2.20(4) | 2.748(2) | 127(4) |
| O9-H9A⋯O8 | 0.80(3) | 1.92(3) | 2.712(3) | 169(3) |
| O9-H9B⋯O9 g | 0.81(2) | 1.94(5) | 2.753(2) | 177(7) |
| O9-H9C⋯O4 | 0.80(2) | 2.07(2) | 2.849(2) | 163(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Głowniak, I.; Wolf, W.M. Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline. Crystals 2020, 10, 97. https://doi.org/10.3390/cryst10020097
Gacki M, Kafarska K, Pietrzak A, Korona-Głowniak I, Wolf WM. Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline. Crystals. 2020; 10(2):97. https://doi.org/10.3390/cryst10020097
Chicago/Turabian StyleGacki, Michał, Karolina Kafarska, Anna Pietrzak, Izabela Korona-Głowniak, and Wojciech M. Wolf. 2020. "Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline" Crystals 10, no. 2: 97. https://doi.org/10.3390/cryst10020097
APA StyleGacki, M., Kafarska, K., Pietrzak, A., Korona-Głowniak, I., & Wolf, W. M. (2020). Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline. Crystals, 10(2), 97. https://doi.org/10.3390/cryst10020097