Supramolecular Architecture in a Ni(II) Complex with a Weakly Bonded N,N′-(1,4-phenylenedi- carbonyl)Diglycinate Counter-Anion: Crystal Structure Investigation and Hirshfeld Surface Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Matrials and Synthesis of the N,N′-(1,4-Phenylenedicarbonyl)Diglycine
2.2. Preparation of the Ni(II) Complex
2.3. X-ray Crystallography
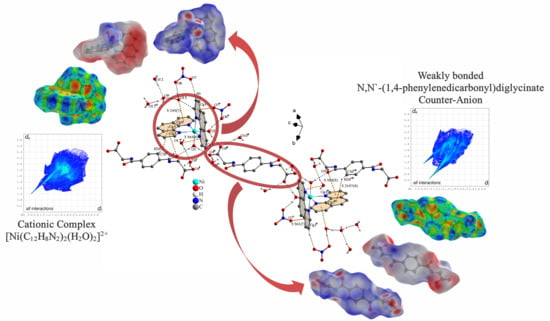
3. Results and Discussion
3.1. Description of the Crystal Structure
3.2. Hirshfeld Surface Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werner, A. Beitrag zur Konstitution anorganischer Verbindungen. Z. Anorg. Allg. Chem. 1895, 9, 382–417. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Coordination chemistry: The scientific legacy of Alfred Werner. Chem. Soc. Rev. 2013, 42, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1999, 1, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Ogba, O.M.; Warner, N.C.; O’Leary, D.J.; Grubbs, R.H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 2018, 47, 4510–4544. [Google Scholar] [CrossRef] [PubMed]
- Biefeld, C.G.; Eick, H.A.; Grubbs, R.H. Crystal structure of bis(triphenylphosphine)tetramethyleneplatinum(II). Inorg. Chem. 1973, 12, 2166–2170. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Deo, K.M.; Ang, D.L.; McGhie, B.; Rajamanickam, A.; Dhiman, A.; Khoury, A.; Holland, J.; Bjelosevic, A.; Pages, B.; Gordon, C.; et al. Platinum coordination compounds with potent anticancer activity. Coord. Chem. Rev. 2018, 375, 148–163. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Foster, M.E.; Léonard, F.; Stavila, V.; Feng, P.L.; Doty, F.P.; Leong, K.; Ma, E.Y.; Johnston, S.R.; Talin, A.A. Guest-Induced Emergent Properties in Metal–Organic Frameworks. J. Phys. Chem. Lett. 2015, 6, 1182–1195. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Doty, F.P.; Bauer, C.A.; Skulan, A.J.; Grant, P.G.; Allendorf, M.D. Scintillating Metal-Organic Frameworks: A New Class of Radiation Detection Materials. Adv. Mater. 2009, 21, 95–101. [Google Scholar] [CrossRef]
- Perry, J.J., IV; Feng, P.L.; Meek, S.T.; Leong, K.; Doty, F.P.; Allendorf, M.D. Connecting structure with function in metal-organic frameworks to design novel photo- and radioluminescent materials. J. Mater. Chem. 2012, 22, 10235. [Google Scholar] [CrossRef]
- Pook, N.-P.; Fruhner, C.-J.; Franzl, T.; Denzer, U.; Adam, A. Instrumentation for X-ray Excited and Laser Induced Fluorescence Lifetime Spectroscopy Using Two-Dimensional Photon Counting. IEEE Trans. Nuclear Sci. 2012, 59, 2319–2323. [Google Scholar] [CrossRef]
- Pook, N.-P.; Fruhner, C.-J.; Franzl, T.; Denzer, U.; Adam, A. Further performance tests of a picosecond X-ray and laser induced streak camera system with fast scintillation materials. Radiat. Meas. 2013, 56, 281–284. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers; Royal Society of Chemistry: Cambridge, UK, 2008; ISBN 978-0-85404-837-3. [Google Scholar]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Yamada, T.; Otsubo, K.; Makiura, R.; Kitagawa, H. Designer coordination polymers: Dimensional crossover architectures and proton conduction. Chem. Soc. Rev. 2013, 42, 6655–6669. [Google Scholar] [CrossRef]
- Leong, W.L.; Vittal, J.J. One-Dimensional Coordination Polymers: Complexity and Diversity in Structures, Properties, and Applications. Chem. Rev. 2011, 111, 688–764. [Google Scholar] [CrossRef]
- Schneider, H.-J. Binding Mechanisms in Supramolecular Complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal-Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Brenig, C.; Prieto, L.; Oetterli, R.; Zelder, F. A Nickel(II)-Containing Vitamin B12 Derivative with a Cofactor-F430-type π-System. Angew. Chem. Int. Ed Engl. 2018, 57, 16308–16312. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Nickel-based Enzyme Systems. J. Biol. Chem. 2009, 284, 18571–18575. [Google Scholar] [CrossRef] [PubMed]
- Lawless, C.; Pearson, R.D.; Selley, J.N.; Smirnova, J.B.; Grant, C.M.; Ashe, M.P.; Pavitt, G.D.; Hubbard, S.J. Upstream sequence elements direct post-transcriptional regulation of gene expression under stress conditions in yeast. BMC Genom. 2009, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, B.; Musiani, F.; Benini, S.; Ciurli, S. Chemistry of Ni2+ in urease: Sensing, trafficking, and catalysis. Acc. Chem. Res. 2011, 44, 520–530. [Google Scholar] [CrossRef]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef]
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic Rings in Chemical and Biological Recognition: Energetics and Structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef]
- Duan, J.; Zheng, B.; Bai, J.; Zhang, Q.; Zuo, C. Metal-dependent dimensionality in coordination polymers of a semi-rigid dicarboxylate ligand with additional amide groups: Syntheses, structures and luminescent properties. Inorg. Chim. Acta 2010, 363, 3172–3177. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Casella, L.; Hadjiliadis, N.; Monzani, E.; Kourkoumelis, N.; Plakatouras, J.C. Interpenetrated networks from a novel nanometer-sized pseudopeptidic ligand, bridging water, and transition metal ions with cds topology. Chem. Commun. 2005, 30, 3859–3861. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Casella, L.; Boudalis, A.K.; Monzani, E.; Plakatouras, J.C. Structural variation from 1D chains to 3D networks: A systematic study of coordination number effect on the construction of coordination polymers using the terepthaloylbisglycinate ligand. New J. Chem. 2011, 35, 1060–1071. [Google Scholar] [CrossRef]
- Zhang, H.-T.; You, X.-Z. The one-dimensional zigzag coordination polymer catena -poly[[[triaquazinc(II)]-μ-N,N′-(benzene-1,4-dicarboxamido)diacetato-κ2O:O′] dihydrate]. Acta Crystallogr. 2005, E61, 1163–1165. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Li, Y.-Z.; Wang, T.-W.; Nfor, E.N.; Wang, H.-Q.; You, X.-Z. A ZnII-Based Chiral Crystalline Nanotube. Eur. J. Inorg. Chem. 2006, 17, 3532–3536. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Berryman, O.B.; Johnson, D.W. Experimental evidence for interactions between anions and electron-deficient aromatic rings. Chem. Commun. 2009, 22, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Pook, N.-P.; Gjikaj, M.; Adam, A. Bis[bis(2,2′-bipyridine-κ2N,N′)(carbonato-κ2O,O′)cobalt(III)] 2-{4-[(carboxylatomethyl)carbamoyl]benzamido}acetatehexahydrate. Acta Crystallogr. 2014, E70, 160–161. [Google Scholar] [CrossRef] [Green Version]
- Pook, N.-P.; Hentrich, P.; Gjikaj, M. Crystal structure of bis-tris-(1,10-phenanthroline-κ2N,N’)cobalt(II) tetranitrate N,N’-(1,4-phenylenedicarbonyl)diglycine solvate octahydrate. Acta Crystallogr. 2015, E71, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Pook, N.-P.; Adam, A.; Gjikaj, M. Crystal structure and Hirshfeld surface analysis of (μ-2-{4-(carboxyl-atometh-yl)carbamoylbenzamido}-acetato-κ2O:O’)bis-bis-(1,10-phenanthroline-κ2N,N’)-copper(II) dinitrate N,N’-(1,4-phenylenedicarbonyl)diglycine monosolvateoctahydrate. Acta Crystallogr. 2019, E75, 667–674. [Google Scholar] [CrossRef]
- Cleaver, C.S.; Pratt, B.C. Synthesis of 2,2’-Bis-[5(4H)-oxazolones]. J. Am. Chem. Soc. 1955, 77, 1544–1546. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Köse, D.A.; Zümreoglu-Karan, B.; Koşar, B.; Büyükgüngör, O. Diaquabis(phen)Ni(II) Complex with Vitamin B13 Counter-ions. J. Chem. Crystallogr. 2008, 38, 305–309. [Google Scholar] [CrossRef]
- Prior, T.J.; Rujiwatra, A.; Chimupala, Y. [Ni(1,10-phenanthroline)2(H2O)2](NO3)2: A Simple Coordination Complex with a Remarkably Complicated Structure that Simplifies on Heating. Crystals 2011, 1, 178–194. [Google Scholar] [CrossRef] [Green Version]
- SciFinder. Chemical Abstracts Service: Columbus, OH, 2010; A Substructure Search with Chemical Structure Editor. Available online: https://scifinder.cas.org (accessed on 2 November 2019).
- Jorgensen, W.L.; Pranata, J. Importance of secondary interactions in triply hydrogen bonded complexes: Guanine-cytosine vs uracil-2,6-diaminopyridine. J. Am. Chem. Soc. 1990, 112, 2008–2010. [Google Scholar] [CrossRef]
- Van der Lubbe, S.C.C.; Zaccaria, F.; Sun, X.; Guerra, C.F. Secondary Electrostatic Interaction Model Revised: Prediction Comes Mainly from Measuring Charge Accumulation in Hydrogen-Bonded Monomers. J. Am. Chem. Soc. 2019, 141, 4878–4885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, S.-I.; Uchimaru, T. Secondary Interaction Contribution in Hydrogen-Bonded Complex: Theoretical Model Study in Hydrogen Fluoride Trimer. J. Comput. Chem. Jpn. 2004, 3, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, S.C.; Murray, T.J. Hydrogen bonded complexes with the AA·DD, AA·DDD, and AAA·DD motifs: The role of three centered (bifurcated) hydrogen bonding. Tetrahedron Lett. 1994, 35, 4077–4080. [Google Scholar] [CrossRef]
- Barceló-Oliver, M.; Terrón, A.; García-Raso, A.; Lah, N.; Turel, I. Intermolecular C–H·π interactions in 1,5-diphenyl-3-(2-pyridyl)-2-pyrazoline. Acta Crystallogr. 2010, C66, 313–316. [Google Scholar] [CrossRef]
- Kumar Seth, S.; Dey, B.; Kar, T.; Mukhopadhyay, S. Experimental observation of supramolecular carbonyl–π/π–π/π–carbonyl assemblies of CuII complex of iminodiacetate and dipyridylamine. J. Mol. Struct. 2010, 973, 81–88. [Google Scholar] [CrossRef]
- Egli, M.; Sarkhel, S. Lone Pair—Aromatic Interactions: To Stabilize or Not to Stabilize. Acc. Chem. Res. 2007, 40, 197–205. [Google Scholar] [CrossRef]
- Gao, X.-L.; Lu, L.-P.; Zhu, M.-L. The crucial role of C–H…O and C=O…π interactions in the building of three-dimensional structures of dicarboxylic acid–biimidazole compounds. Acta Crystallogr. 2009, C65, o123–o127. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P.; Reedijk, J. Lone pair–π interactions: A new supramolecular bond? CrystEngComm 2008, 10, 1501–1515. [Google Scholar] [CrossRef]
- Wan, C.-Q.; Chen, X.-D.; Mak, T.C.W. Supramolecular frameworks assembled via intermolecular lone pair-aromatic interaction between carbonyl and pyridyl groups. CrystEngComm 2008, 10, 475–478. [Google Scholar] [CrossRef]
- Jain, A.; Ramanathan, V.; Sankararamakrishnan, R. Lone pair π interactions between water oxygens and aromatic residues: Quantum chemical studies based on high-resolution protein structures and model compounds. Protein Sci. 2009, 18, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, P.; Seth, S.K.; Das, A.; Hemming, J.; Prendergast, R.; Helliwell, M.; Choudhury, S.R.; Frontera, A.; Mukhopadhyay, S. Anion Induced Formation of Supramolecular Associations Involving Lone pair−π and Anion−π Interactions in Co(II) Malonate Complexes: Experimental Observations, Hirshfeld Surface Analyses and DFT Studies. Inorg. Chem. 2012, 51, 3557–3571. [Google Scholar] [CrossRef] [PubMed]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-π interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef]
- Gamez, P.; Mooibroek, T.J.; Teat, S.J.; Reedijk, J. Anion Binding Involving π-Acidic Heteroaromatic Rings. Acc. Chem. Res. 2007, 40, 435–444. [Google Scholar] [CrossRef]
- Jia, C.; Miao, H.; Hay, B.P. Crystal Structure Evidence for the Directionality of Lone Pair−π interactions: Fact or Fiction? Cryst. Growth Des. 2019, 19, 6806–6821. [Google Scholar] [CrossRef] [Green Version]
- Gathergood, N.; Scammells, P.J.; Fallon, G.D. Inter- and intramolecular C–H·π interactions in morphine bis(1-naphthoate). Acta Crystallogr. 2003, C59, 485–487. [Google Scholar] [CrossRef] [Green Version]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130. [Google Scholar] [CrossRef]
- Plevin, M.J.; Bryce, D.L.; Boisbouvier, J. Direct detection of CH/π interactions in proteins. Nat. Chem. 2010, 2, 466–471. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 3.1; University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Jayatilaka, D.; Grimwood, D.J.; Lee, A.; Lemay, A.; Russel, A.J.; Taylor, C.; Wolff, S.K.; Cassam-Chenai, P.; Whitton, A. TONTO—A System for Computational Chemistry. Available online: http://hirshfeldsurface.net/ (accessed on 22 November 2019).








| Compound | [Ni(C12H8N2)2(H2O)2]2·(C12H10N2O6)·(NO3)2·10H2O |
|---|---|
| Empirical formula | C30H35N6NiO13 |
| Formula weight | 746.35 |
| Temperature (K) | 223 |
| Diffractometer | Stoe IPDS II |
| Wavelength (Å) | 0.71073 |
| Crystal system | triclinic |
| Space group | Pī (No. 2) |
| a, b, c (Å) | 12.5801(14), 12.5852(16), 12.6357(14) |
| α, β, γ (°) | 114.670(9), 109.175(8), 95.749(10) |
| V (Å3) | 1650.2(4) |
| Z | 2 |
| Dcalc (g·cm3) | 1.502 |
| µ (mm-1) | 0.664 |
| F(000) | 778 |
| Crystal size (mm) | 0.22 × 0.21 × 0.20 |
| θ Range (°) | 1.94–25.25 |
| Index ranges | −15 ≤ h ≤ 15 −15 ≤ k ≤ 15 −15 ≤ l ≤ 15 |
| Reflection collected/unique | 15380/5887 |
| Completeness to θ (%) | 98.5 |
| Absorption correction | Numerical; X-AREA, X-RED (2008) |
| Max. and min. transmission | 0.5023, 0.7193 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/parameters | 5887/496 |
| Goodness-of-fit on F2 | 1.031 |
| R1 [I ≥ 2σ(I)]/R1 (all data) | 0.0528/0.0615 |
| wR2 [I ≥ 2σ(I)]/wR2 (all data) | 0.1406/0.1467 |
| Largest diff. peak and hole (e·Å3) | 1.30, –0.58 |
| Deposition number | CCDC 1957420 |
| Bond | Lengths | Bond | Lengths |
| Ni–N1 | 2.095 (3) | Ni–N2 | 2.083 (3) |
| Ni–N3 | 2.101 (3) | Ni–N4 | 2.084 (3) |
| Ni–O1 | 2.088 (3) | Ni–O2 | 2.047 (2) |
| Bond | Angle | Bond | Angle |
| N1–Ni–N2 | 80.01 (11) | N2–Ni–N3 | 96.98 (11) |
| N3–Ni–N4 | 79.94 (11) | N4–Ni–N2 | 173.16 (11) |
| N1–Ni–N4 | 94.08 (12) | N1–Ni–N3 | 94.39 (12) |
| O1–Ni–N1 | 171.38 (11) | O1–Ni–N2 | 91.39 (11) |
| O1–Ni–N3 | 87.24 (10) | O1–Ni–N4 | 94.55 (11) |
| O2–Ni–N1 | 93.87 (3) | O2–Ni–N2 | 90.89 (12) |
| O2–Ni–N3 | 169.47 (9) | O2–Ni–N4 | 92.96 (12) |
| O1–Ni–O2 | 85.58 (10) |
| D—H···A | d(D—H) | d(H···A) | d(D···A) | ∠D—H···A |
|---|---|---|---|---|
| O1—H1A···O4iii | 0.87 (5) | 1.82 (5) | 2.692 (3) | 175 (4) |
| O1—H1B···O5ii | 0.74 (5) | 2.00 (5) | 2.741 (3) | 173 (4) |
| O2—H2A···O6iv | 0.84 (2) | 1.91 (2) | 2.739 (5) | 166 (5) |
| O2—H2A···O7iv | 0.84 (2) | 2.56 (4) | 3.258 (6) | 141 (4) |
| O2—H2A···N6iv | 0.84 (2) | 2.57 (2) | 3.389 (4) | 166 (5) |
| O2—H2B···O3iii | 0.82 (5) | 1.84 (5) | 2.658 (3) | 173 (5) |
| O9—H9A···O12xi | 0.79 (1) | 2.05 (3) | 2.787 (5) | 154 (7) |
| O9—H9B···O4xv | 0.90 (2) | 2.07 (3) | 2.937 (4) | 159 (6) |
| O10—H10A···O6i | 0.83 (7) | 2.07 (7) | 2.824 (6) | 151 (7) |
| O10—H10B···O3 | 0.76 (8) | 2.13 (8) | 2.867 (5) | 162 (7) |
| O11—H11A···O10 | 0.91 (8) | 1.88 (8) | 2.787 (6) | 172 (7) |
| O11—H11B···O8v | 0.88 (8) | 2.20 (8) | 3.056 (6) | 163 (7) |
| O11—H11B···N6v | 0.88 (8) | 2.61 (8) | 3.473 (5) | 165 (6) |
| O12—H12A···O11 | 0.89 (2) | 1.88 (2) | 2.769 (5) | 173 (7) |
| O12—H12B···O9v | 0.88 (2) | 1.97 (2) | 2.837 (5) | 168 (7) |
| O13—H13A···O8 | 1.01 (2) | 1.94 (3) | 2.942 (6) | 169 (8) |
| O13—H13A···N6 | 1.01 (2) | 2.65 (4) | 3.610 (6) | 158 (7) |
| O13—H13B···O9 | 0.97 (2) | 1.96 (5) | 2.842 (6) | 149 (8) |
| N5—H5···O8i | 0.85 (4) | 2.07 (4) | 2.900 (5) | 164 (4) |
| C1—H1···O13 | 0.94 | 2.53 | 3.294 (5) | 139 |
| C2—H2···O7 | 0.94 | 2.55 | 3.469 (7) | 166 |
| C10—H10···O1 | 0.94 | 2.63 | 3.156 (4) | 116 |
| C13—H13···O12 | 0.94 | 2.54 | 3.415 (5) | 156 |
| Y—X···Cg | d(X···Cg) | d(Y···Cg) | ∠Y—X···Cg |
| C3—H3···Cg7 | 3.4215(5) | 3.925(5) | 115.9(3) |
| C8—H8···Cg1iii | 3.3517(7) | 3.735(3) | 106.9(3) |
| C15—H15···Cg1xi | 2.7372(5) | 3.668(3) | 170.3(3) |
| C26—H26···Cg5xv | 3.2107(4) | 3.912(4) | 132.9(2) |
| C28—O5···Cg4xv | 3.593(3) | 3.581(4) | 78.5(2) |
| N6—O7···Cg1iv | 3.561(7) | 4.616(4) | 147.3(4) |
| O12···Cg3xvi | 3.365(4) | ||
| O13···Cg6 | 3.245(7) | ||
| Cg···Cg | d(Cg···Cg) | ||
| Cg2···Cg3iii | 3.6649(7) | ||
| Cg4···Cg4xi | 3.5600(4) | ||
| Cg6···Cg6vi | 3.5628(4) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pook, N.-P. Supramolecular Architecture in a Ni(II) Complex with a Weakly Bonded N,N′-(1,4-phenylenedi- carbonyl)Diglycinate Counter-Anion: Crystal Structure Investigation and Hirshfeld Surface Analysis. Crystals 2019, 9, 615. https://doi.org/10.3390/cryst9120615
Pook N-P. Supramolecular Architecture in a Ni(II) Complex with a Weakly Bonded N,N′-(1,4-phenylenedi- carbonyl)Diglycinate Counter-Anion: Crystal Structure Investigation and Hirshfeld Surface Analysis. Crystals. 2019; 9(12):615. https://doi.org/10.3390/cryst9120615
Chicago/Turabian StylePook, Niels-Patrick. 2019. "Supramolecular Architecture in a Ni(II) Complex with a Weakly Bonded N,N′-(1,4-phenylenedi- carbonyl)Diglycinate Counter-Anion: Crystal Structure Investigation and Hirshfeld Surface Analysis" Crystals 9, no. 12: 615. https://doi.org/10.3390/cryst9120615
APA StylePook, N. -P. (2019). Supramolecular Architecture in a Ni(II) Complex with a Weakly Bonded N,N′-(1,4-phenylenedi- carbonyl)Diglycinate Counter-Anion: Crystal Structure Investigation and Hirshfeld Surface Analysis. Crystals, 9(12), 615. https://doi.org/10.3390/cryst9120615