Linear One-Dimensional Coordination Polymers Constructed by Dirhodium Paddlewheel and Tetracyanido-Metallate Building Blocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis
Synthesis of (PPh4)2n[{Rh2(µ-O2CCH3)4}{M(CN)4}]n (M = Ni (1), Pd(2), Pt(3))
3. Results and Discussion
3.1. Synthesis, Spectroscopic Characterization, and Spectrometric Characterization
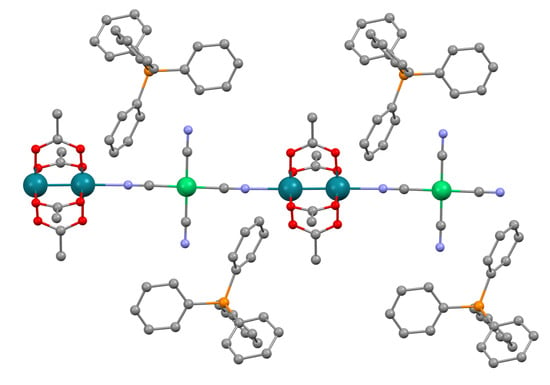
3.2. Structural Description
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Liddle, S.T. (Ed.) Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Hui, B.C.Y.; Teo, W.K.; Rempel, G.L. Activation of hydrogen by bridged transition metal carboxylates. Rhodium(II) acetate catalyzed hydrogenation of olefins. Inorg. Chem. 1973, 12, 757–762. [Google Scholar] [CrossRef]
- Hubert, A.J.; Noels, A.F.; Anciaux, A.J.; Teyssié, P. Rhodium(II) carboxylates: Novel highly efficient catalysts for the cyclopropanation of alkenes with alkyl diazoacetates. Synthesis 1976, 9, 600–602. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 2019, 3, 347–360. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Yang, J.-M.; Zhao, Y.-T.; Zhu, S.-F. Rhodium-Catalyzed Si−H Bond Insertion Reactions Using Functionalized Alkynes as Carbene Precursors. ACS Catal. 2019, 9, 5353–5357. [Google Scholar] [CrossRef]
- Adly, F.G.; Gardiner, M.G.; Ghanem, A. Design and Synthesis of Novel Chiral Dirhodium(II) Carboxylate Complexes for Asymmetric Cyclopropanation Reactions. Chem. Eur. J. 2016, 22, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, E.; Berto, T.C.; Berry, J.F. Axial Ligand Coordination to the C−H Amination Catalyst Rh2(esp)2: A Structural and Spectroscopic Study. Inorg. Chem. 2015, 54, 8817–8824. [Google Scholar] [CrossRef] [PubMed]
- Mukhacheva, A.A.; Volchek, V.V.; Abramov, P.A.; Sokolov, M.N. Blocking {RhCl}2+ disorder in the crystal structure of a [SiW11O39{RhCl}]6− salt: Direct localization of the heterometal in a monosubstituted Keggin anion. Inorg. Chem. Commun. 2018, 89, 10–12. [Google Scholar] [CrossRef]
- Enriquez Garcia, A.; Lai, B.; Gopinathan, S.G.; Harris, H.H.; Shemanko, C.S.; Jalilehvand, F. Nuclear localization of dirhodium(II) complexes in breast cancer cells by X-ray fluorescence microscopy. Chem. Commun. 2019, 55, 8223–8226. [Google Scholar] [CrossRef]
- Lewis, J.C. Beyond the Second Coordination Sphere: Engineering Dirhodium Artificial Metalloenzymes To Enable Protein Control of Transition Metal Catalysis. Acc. Chem. Res. 2019, 52, 576–584. [Google Scholar] [CrossRef]
- Masternak, J.; Gilewska, A.; Kazimierczuk, K.; Khavryuchenko, O.V.; Wietrzyk, J.; Trynda, J.; Barszcz, B. Synthesis, physicochemical and theoretical studies on new rhodium and ruthenium dimers. Relationship between structure and cytotoxic activity. Polyhedron 2018, 154, 263–274. [Google Scholar] [CrossRef]
- Knoll, J.D.; Turro, C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 2015, 282–283, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Belman, O.F.; Varela, Y.; Flores-Álamo, M.; Wrobel, K.; Gutierrez-Granados, S.; Peralta-Hernández, J.M.; Jiménez-Halla, J.O.C.; Serrano, O. Microwave-Assisted Synthesis and Characterization of [Rh2(OAc)4(L)2] Paddlewheel Complexes: A Joint Experimental and Computational Study. Int. J. Inorg. Chem. 2017, 2017, 5435436. [Google Scholar] [CrossRef]
- Ye, Q.-S.; Li, X.-N.; Jin, Y.; Yu, J.; Chang, Q.-W.; Jiang, J.; Yan, C.-X.; Li, J.; Liu, W.-P. Synthesis, crystal structures and catalytic activity of tetrakis(acetato)dirhodium(II) complexes with axial picoline ligands. Inorg. Chim. Acta 2015, 434, 113–120. [Google Scholar] [CrossRef]
- Cmoch, P.; Głaszczka, R.; Jaźwiński, J.; Kamieński, B.; Senkara, E. Adducts of nitrogenous ligands with rhodium(II) tetracarboxylates and tetraformamidinate: NMR spectroscopy and density functional theory calculations. Magn. Reson. Chem. 2014, 52, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Milaeva, E.R.; Meleshonkova, N.N.; Shpakovsky, D.B.; Uspensky, K.V.; Dolganov, A.V.; Magdesieva, T.V.; Fionov, A.V.; Sidorov, A.A.; Aleksandrov, G.-G.; Eremenko, I.L. Synthesis and redox properties of dinuclear rhodium(II) carboxylates with 2,6-di-tert-butylphenol moieties. Inorg. Chim. Acta 2010, 363, 1455–1461. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Shimodaira, T.; Yan, Y.-N.; Yamasaki, M.; Tanaka, H.; Omata, K.; Kawamoto, T.; Handa, M. Paddlewheel-Type Dirhodium Tetrapivalate Based Coordination Polymer: Synthesis, Characterization, and Self-Assembly and Disassembly Transformation Properties. Eur. J. Inorg. Chem. 2016, 2810–2815. [Google Scholar] [CrossRef]
- Fritsch, N.; Wick, C.R.; Waidmann, T.; Dral, P.O.; Tucher, J.; Heinemann, F.W.; Shubina, T.E.; Clark, T.; Burzlaff, N. Multiply Bonded Metal(II) Acetate (Rhodium, Ruthenium, and Molybdenum) Complexes with the trans-1,2-Bis(N-methylimidazol-2-yl)ethylene Ligand. Inorg. Chem. 2014, 53, 12305–12314. [Google Scholar] [CrossRef]
- Dikarev, E.V.; Shpanchenko, R.V.; Andreini, K.W.; Block, E.; Jin, J.; Petrukhina, M.A. Powder Diffraction Study of a Coordination Polymer Comprised of Rigid Building Blocks: [Rh2(O2CCH3)4·μ2-Se2C5H8-Se,Se’]∞. Inorg. Chem. 2004, 43, 5558–5563. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.-J.; Lough, A.J. New dirhodium(II,II) carboxylates with 2,6-bis(N’-1,2,4-triazolyl)pyridinato ligand (btp). Polyhedron 2001, 20, 3073–3078. [Google Scholar] [CrossRef]
- Heyduk, A.F.; Krodel, D.J.; Meyer, E.E.; Nocera, D.G. A Luminescent Heterometallic Dirhodium-Silver Chain. Inorg. Chem. 2002, 41, 634–636. [Google Scholar] [CrossRef]
- Uemura, K. One-dimensional complexes extended by unbridged metal–metal bonds based on a HOMO–LUMO interaction at the dz2 orbital between platinum and heterometal atoms. Dalton Trans. 2017, 46, 5474–5492. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.N.; Adonin, S.A.; Peresypkina, E.V.; Abramov, P.A.; Smolentsev, A.I.; Potemkin, D.I.; Snytnikov, P.V.; Fedin, V.P. Reactions of rhodium (II) acetate with non-lacunary Keggin and Dawson polyoxoanions and related catalytic studies. Inorg. Chim. Acta 2013, 394, 656–662. [Google Scholar] [CrossRef]
- Adly, F.G.; Bollard, H.; Gardiner, M.G.; Ghanem, A. Chiral Dirhodium(II) Carboxylates: New Insights into the Effect of Ligand Stereo-Purity on Catalyst Structure and Enantioselectivity. Catalysts 2018, 8, 268. [Google Scholar] [CrossRef]
- Uemura, K.; Ebihara, M. One-Dimensionally Extended Paddlewheel Dirhodium Complexes from Metal–Metal Bonds with Diplatinum Complexes. Inorg. Chem. 2011, 50, 7919–7921. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Kanbara, T.; Ebihara, M. Two Types of Heterometallic One-Dimensional Alignment Composed of Acetamidate-Bridged Dirhodium and Pivalamidate-Bridged Diplatinum Complexes. Inorg. Chem. 2014, 53, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Yamada, T.; Kanbara, T.; Ebihara, M. Acetamidate-bridged paddlewheel dirhodium complex sandwiched by mononuclear platinum complexes with axial metal–metal bonds affording neutral heterometallic one-dimensional alignments. Inorg. Chim. Acta 2015, 424, 194–201. [Google Scholar] [CrossRef]
- Yamada, T.; Ebihara, M.; Uemura, K. Heterometallic one-dimensional chain with tetradeca metal repetition constructed by amidate bridged dirhodium and pivalate bridged diplatinum complexes influenced by hydrogen bonding. Dalton Trans. 2016, 45, 12322–12328. [Google Scholar] [CrossRef]
- Uemura, K.; Ebihara, M. Paramagnetic One-Dimensional Chains Comprised of Trinuclear Pt–Cu–Pt and Paddlewheel Dirhodium Complexes with Metal–Metal Bonds. Inorg. Chem. 2013, 52, 5535–5550. [Google Scholar] [CrossRef]
- Uemura, K. Magnetic behavior in heterometallic one-dimensional chains or octanuclear complex regularly aligned with metal-metal bonds as –Rh-Rh-Pt-Cu-Pt. J. Mol. Struct. 2018, 1162, 31–36. [Google Scholar] [CrossRef]
- Amo-Ochoa, P.; Delgado, S.; Gallego, A.; Gómez-García, C.J.; Jiménez-Aparicio, R.; Martínez, G.; Perles, J.; Torres, M.R. Structure and Properties of One-Dimensional Heterobimetallic Polymers Containing Dicyanoaurate and Dirhodium(II) Fragments. Inorg. Chem. 2012, 51, 5844–5849. [Google Scholar] [CrossRef]
- Cruz, P.; Fernandez-Bartolome, E.; Cortijo, M.; Delgado-Martínez, P.; González-Prieto, R.; Priego, J.L.; Torres, M.R.; Jiménez-Aparicio, R. Synthesis and Structural Characterization of a Series of One-Dimensional Heteronuclear Dirhodium-Silver Coordination Polymers. Polymers 2019, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, E.V.; Virovets, A.V.; Blatov, V.A.; Peresypkina, E.V. Topological Motifs in Cyanometallates: From Building Units to Three-Periodic Frameworks. Chem. Rev. 2015, 115, 12286–12319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, F.; del Barco, E.; Fishman, R.S.; Miller, J.S. Low temperature hysteretic behavior of the interpenetrating 3-D network structured [Ru2(O2CMe)4]3[Fe(CN)6] magnet. Polyhedron 2013, 64, 73–76. [Google Scholar] [CrossRef]
- Kennon, B.S.; Stone, K.H.; Stephens, P.W.; Miller, J.S. Interpenetrating diruthenium tetraformate monocation, [RuII/III2(O2CH)4]+, based 3-D molecule-based magnets. CrystEngComm 2009, 11, 2185–2191. [Google Scholar] [CrossRef]
- Qin, Y.-L.; Yang, B.-W.; Wang, G.-F.; Sun, H. A cyanide-bridged heterometallic coordination polymer constructed from square-planar [Ni(CN)4]2-: Synthesis, crystal structure, thermal decomposition, electron paramagnetic resonance (EPR) spectrum and magnetic properties. Acta Cryst. 2016, C72, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Gör, K.; Tursun, M.; Keşan, G.; Kürkçüoğlu, G.S.; Rhyman, L.; Parlak, C.; Ramasami, P.; Yeşilel, O.Z.; Büyükgüngör, O. Novel Cyanide-Bridged Heterometallic Two-Dimensional Complex of 3-Methylpyridazine: Synthesis, Crystallographical, Vibrational, Thermal and DFT Studies. J. Inorg. Organomet. Polym. 2015, 25, 1205–1217. [Google Scholar] [CrossRef]
- Manna, S.C.; Ribas, J.; Zangrando, E.; Chaudhuri, N.R. Hetero-metallic frameworks of [Pd(CN)4]2- and Cu(II) with triamines: A rare example of a tetracyanometallate bridged 2D coordination polymer. Polyhedron 2007, 26, 3189–3198. [Google Scholar] [CrossRef]
- Marinescu, G.; Madalan, A.M.; Andruh, M. New heterometallic coordination polymers based on zinc(II) complexes with Schiff-base ligands and dicyanometallates: Synthesis, crystal structures, and luminescent properties. J. Coord. Chem. 2015, 68, 479–490. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Supramolecular Motifs: Concerted Multiple Phenyl Embraces between Ph4P+ Cations Are Attractive and Ubiquitous. Chem. Eur. J. 1996, 2, 481–486. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Concerted supramolecular motifs: Linear columns and zigzag chains of multiple phenyl embraces involving Ph4P+ cations in crystals. J. Chem. Soc. Dalton Trans. 1996, 3755–3769. [Google Scholar] [CrossRef]
- Ali, B.; Dance, I.; Scudder, M.; Craig, D. Dimorphs of (Ph4P)2[Cd2(SPh)6]: Crystal Packing Analyses and the Interplay of Intermolecular and Intramolecular Energies. Cryst. Growth Des. 2002, 2, 601–607. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Białek, M.J.; Janczak, J.; Zoń, J.; Dobosz, A. Extending the Family of Tetrahedral Tectons: Phenyl Embraces in Supramolecular Polymers of Tetraphenylmethane-based Tetraphosphonic Acid Templated by Organic Bases. Cryst. Growth Des. 2014, 14, 6143–6153. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Yang, Z.; Ebihara, M.; Kawamura, T. One-dimensional chain structures constructed from tetra(acetamidato)dirhodium(II,III) complexes and square planar platinum and palladium complexes. Inorg. Chim. Acta 2006, 359, 2465–2471. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.J.; Nam, W. A ferric-cyanide-bridged one-dimensional dirhodium complex with (18-crown-6) potassium cations. Acta Cryst. 2001, C57, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Harrison, W.T.A.; Jacobson, A.J. Synthesis and crystal structure of the two-dimensional polymer K3Co(CN)6·2Rh2(O2CMe)4. Chem. Commun. 1996, 399–400. [Google Scholar] [CrossRef]
- Park, D.; Song, M.; Ha, S.; Kang, A.-S.; Moon, S.-B.; Ryu, C.-H.; Chung, J.-H. A Novel Cyanide-Bridged Thulium-Nickel Heterobimetallic Polymeric Complex {(H2O)2(DMF)10Tm2[Ni(CN)4]2}[Ni(CN)4] including O-H…N Hydrogen Bond. Bull. Korean Chem. Soc. 2012, 33, 4197–4200. [Google Scholar] [CrossRef] [Green Version]
- Korčok, J.L.; Leznoff, D.B. Thermal expansion of mercury(II) cyanide and HgCN(NO3). Polyhedron 2013, 52, 72–77. [Google Scholar] [CrossRef]


| 1 (M = Ni) | 2 (M = Pd) | 3 (M = Pt) | |
|---|---|---|---|
| Formula | (PPh4)2n[{Rh2(µ-O2CCH3)4}{M(CN)4}]n | ||
| fw | 1283.51 | 1331.21 | 1419.90 |
| Space group | P-1 | P-1 | P21/n |
| a/Å | 12.669(1) | 12.636(1) | 13.0959(4) |
| b/Å | 12.8404(8) | 13.095(2) | 11.7615(4) |
| c/Å | 14.640(1) | 14.524(2) | 25.132(1) |
| α/° | 84.405(5) | 85.137(9) | 90 |
| β/° | 67.765(7) | 68.479(9) | 101.613(4) |
| γ/° | 70.795(7) | 70.436(9) | 90 |
| V/Å3 | 2080.7(3) | 2104.7(4) | 3791.8(2) |
| Z | 1 | 1 | 2 |
| d calc/g·cm−3 | 1.024 | 1.052 | 1.244 |
| μ/mm−1 | 0.694 | 0.676 | 2.356 |
| R indices (I > 2σ(I)) | R1 = 0.0740 wR2 = 0.2276 | R1 = 0.0662 wR2 = 0.1699 | R1 = 0.0395 wR2 = 0.1046 |
| GooF on F2 | 1.017 | 1.005 | 1.008 |
| 1 (M = Ni) | 2 (M = Pd) | 3 (M = Pt) | |
|---|---|---|---|
| Rh-Rh | 2.394(1) | 2.3968(9) | 2.4007(8) |
| Rh-N | 2.275(6) | 2.246(5) | 2.209(5) |
| Rh-O | 2.019(5) 2.024(5) 2.035(5) 2.041(5) | 2.020(4) 2.031(4) 2.040(4) 2.052(4) | 2.026(4) 2.029(4) 2.035(4) 2.042(4) |
| M-C5 (bridge CN) | 1.888(8) | 2.080(6) | 1.998(6) |
| M-C6 (terminal CN) | 1.850(8) | 1.960(8) | 1.979(8) |
| C5-N1 (bridge CN) | 1.062(9) | 1.031(7) | 1.144(8) |
| C6-N2 (terminal CN) | 1.14(1) | 1.151(9) | 1.127(9) |
| Rh-Rh-N | 178.4(2) | 179.1(1) | 178.2(1) |
| Rh-N-C | 178.0(8) | 172.5(5) | 175.8(5) |
| C5-M-C6 | 88.8(3) 91.2(3) | 88.9(2) 91.1(2) | 89.9(3) 90.1(3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prior, D.; Cortijo, M.; González-Prieto, R.; Herrero, S.; Jiménez-Aparicio, R.; Perles, J.; Priego, J.L. Linear One-Dimensional Coordination Polymers Constructed by Dirhodium Paddlewheel and Tetracyanido-Metallate Building Blocks. Crystals 2019, 9, 614. https://doi.org/10.3390/cryst9120614
Prior D, Cortijo M, González-Prieto R, Herrero S, Jiménez-Aparicio R, Perles J, Priego JL. Linear One-Dimensional Coordination Polymers Constructed by Dirhodium Paddlewheel and Tetracyanido-Metallate Building Blocks. Crystals. 2019; 9(12):614. https://doi.org/10.3390/cryst9120614
Chicago/Turabian StylePrior, David, Miguel Cortijo, Rodrigo González-Prieto, Santiago Herrero, Reyes Jiménez-Aparicio, Josefina Perles, and José Luis Priego. 2019. "Linear One-Dimensional Coordination Polymers Constructed by Dirhodium Paddlewheel and Tetracyanido-Metallate Building Blocks" Crystals 9, no. 12: 614. https://doi.org/10.3390/cryst9120614
APA StylePrior, D., Cortijo, M., González-Prieto, R., Herrero, S., Jiménez-Aparicio, R., Perles, J., & Priego, J. L. (2019). Linear One-Dimensional Coordination Polymers Constructed by Dirhodium Paddlewheel and Tetracyanido-Metallate Building Blocks. Crystals, 9(12), 614. https://doi.org/10.3390/cryst9120614