Optical and Thermoelectric Properties of Surface-Oxidation Sensitive Layered Zirconium Dichalcogenides ZrS2−xSex (x = 0, 1, 2) Crystals Grown by Chemical Vapor Transport
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure and Composition Analysis of ZrS2−xSex (x = 0, 1, and 2) Crystals
3.2. Raman Spectroscopy
3.3. Optical Band Gap and Valence-Band Structure
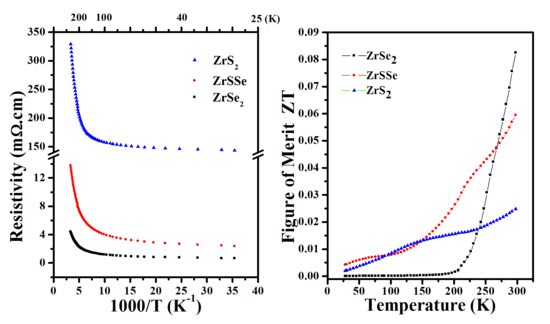
3.4. Transport and Thermoelectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chuang, S.; Chang, T.C.; Takei, K.; Takahashi, T.; Javey, A. High-Performance Single Layered WSe2 p-FETs with Chemically Doped Contacts. Nano Lett. 2012, 12, 3788–3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovchinnikov, D.; Gargiulo, F.; Allain, A.; Pasquier, D.J.; Dumcenco, D.; Ho, C.H.; Yazyev, O.V.; Kis, A. Disorder Engineering and Conductivity Dome in ReS2 with Electrolyte Gating. Nat. Commun. 2016, 7, 12391. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Huang, C.E. Optical Property of the Near Band-Edge Transitions in Rhenium Disulfide and Diselenide. J. Alloys Compd. 2004, 383, 74–79. [Google Scholar] [CrossRef]
- Ho, C.H.; Yen, P.C.; Huang, Y.S.; Tiong, K.K. Photoreflectance Study of the Excitonic Transitions of Rhenium Disulphide Layer Compounds. Phys. Rev. B 2002, 66, 245207. [Google Scholar] [CrossRef]
- Wu, C.C.; Ho, C.H.; Shen, W.T.; Cheng, Z.H.; Huang, Y.S.; Tiong, K.K. Optical Properties of GaSe1−xSx Series Layered Semiconductors Grown by Vertical Bridgman Method. Mater. Chem. Phys. 2004, 88, 313–317. [Google Scholar] [CrossRef]
- Ho, C.H.; Wu, C.C.; Cheng, Z.H. Crystal Structure and Electronic Structure of GaSe1−xSx Series Layered Solids. J. Cryst. Growth 2005, 279, 321–328. [Google Scholar] [CrossRef]
- Ho, C.H. Thickness-Dependent Carrier Transport and Optically Enhanced Transconductance Gain in III-VI Multilayer InSe. 2D Mater. 2016, 3, 025019. [Google Scholar] [CrossRef]
- Ho, C.H.; Lee, H.W.; Wu, C.C. Polarization Sensitive Behaviour of the Band-Edge Transitions in ReS2 and ReSe2 Layered Semiconductors. J. Phys. Condens. Matter 2004, 16, 5937–5944. [Google Scholar] [CrossRef]
- Ho, C.H.; Huang, K.W. Visible Luminescence and Structural Property of GaSe1−xSx (0 ≤ x ≤ 1) Series Layered Crystals. Solid Stat. Commun. 2005, 136, 591–594. [Google Scholar] [CrossRef]
- Ho, C.H.; Chu, Y.J. Bending Photoluminescence and Surface Photovoltaic Effect on Multilayer InSe 2D Microplate Crystals. Adv. Opt. Mater. 2015, 3, 1570–1578. [Google Scholar] [CrossRef]
- Conroy, L.E.; Park, K.C. Electrical Properties of The Group IV Disulfides, Titanium disulfide, Zirconium Disulfide, Hafnium Disulfide and Tin Disulfide. Inorg. Chem. 1968, 7, 459–463. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Joubert, D.P. Optical spectrum and excitons in bulk and monolayer MX2 (M = Zr, Hf; X = S, Se). Phys. Stat. Solidi B 2016, 253, 705. [Google Scholar] [CrossRef]
- Murray, R.B.; Bromley, R.A.; Yoffe, A.D. The Band Structures of Some Transition Metal Dichalcogenides. II. Group IVA; Octahedral Coordination. J. Phys. C Solid Stat. Phys. 1972, 5, 746–758. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Zhang, M.; Cai, C.; Xie, L. Thickness and Temperature Dependent Electrical Properties of ZrS2 Thin Films Directly Grown on Hexagonal Boron Nitride. Nano Res. 2016, 9, 2931–2937. [Google Scholar] [CrossRef]
- Wilson, J.A.; Yoffe, A.D. The Transition Metal Dichalcogenides Discussion and Interpretation of the Observed Optical, Electrical and Structural Properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Qin, D.; Ge, X.J.; Ding, G.; Lu, J.T. Strain-induced Thermoelectric Performance Enhancement of Monolayer ZrSe2. RSC Adv. 2017, 7, 47243–47250. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.K.; Lucero, M.J.; Scuseria, G.E. The indirect to direct band gap transition in multilayered MoS2 as predicted by screened hybrid density functional theory. Appl. Phys. Lett. 2011, 99, 261908. [Google Scholar] [CrossRef]
- Li, Y.; Kang, J.; Li, J. Indirect-to-direct band gap transition of the ZrS2 monolayer by strain: First-principles calculations. RSC Adv. 2014, 4, 7396. [Google Scholar] [CrossRef]
- Lv, H.Y.; Lu, W.J.; Shao, D.F.; Lu, H.Y.; Sun, Y.P. Strain-induced Enhancement in The Thermoelectric Performance of a ZrS2 Monolayer. J. Mater. Chem. C 2016, 4, 4538–4545. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Din, H.U.; Khan, S.A.; Rehman, G.; Bilal, M.; Nguyen, C.V.; Ahmad, I.; Gan, L.Y.; Amin, B. Theoretical Investigation of Electronic Structure and Thermoelectric Properties of MX2 (M = Zr, Hf; X = S, Se) van der Waals Heterostructures. J. Phys. Chem. Solids 2019, 126, 304–309. [Google Scholar] [CrossRef]
- Hou, B.; Jung, S.H.; Zhang, J.; Hong, Y.; Kim, B.S.; Sohn, J.I.; Lee, E.K.; Choi, B.L.; Whang, D.; Cha, S.; et al. Growth of Quantum Dot Coated Core-Shell Anisotropic Nanowires for Improved Thermal and Electronic Transport. Appl. Phys. Lett. 2019, 114, 243104. [Google Scholar] [CrossRef]
- Yazdani, S.; Pettes, M.T. Nanoscale Self-Assembly of Thermoelectric Materials: A Review of Chemistry-Based Approaches. Nanotechnology 2018, 29, 432001. [Google Scholar] [CrossRef] [PubMed]
- Sadia, Y.; Aminov, Z.; Mogilyansky, D.; Gelbstein, Y. Texture anisotropy of higher manganese silicide following arc-melting and hot-pressing. Intermetallics 2016, 68, 71–77. [Google Scholar] [CrossRef]
- Gelbstein, Y. Pb1−xSnxTe Alloys—Application Considerations. J. Electron. Mater. 2011, 40, 533–536. [Google Scholar] [CrossRef]
- Xing, Y. High-efficiency half-Heusler thermoelectric modules enabled by self-propagating synthesis and topologic structure optimization. Energy Environ. Sci. 2019, 12, 3390–3399. [Google Scholar] [CrossRef]
- Rull-Bravo, M.; Moure, A.; Fernandez, J.F.; Mart’ın-Gonz´alez, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2015, 5, 41653–41667. [Google Scholar] [CrossRef]
- Ho, C.H. Optical Study of The Structural Change in ReS2 Single Crystals Using Polarized Thermoreflectance Spectroscopy. Opt. Express 2005, 13, 8–19. [Google Scholar] [CrossRef]
- Patel, S.G.; Agarwal, M.K.; Batra, N.M.; Lakshminarayana, D. Electrical Properties of Zirconium Diselenide Single Crystals Grown by Iodine Transport Method. Bull. Mater. Sci. 1998, 21, 213–217. [Google Scholar] [CrossRef]
- Golub, A.S.; Zubavichus, Y.V.; Slovokhotov, Y.L.; Novikov, Y.N. Single-Layer Dispersions of Transition Metal Dichalcogenides in The Synthesis of Intercalation Compounds. Russ. Chem. Rev. 2003, 72, 123–141. [Google Scholar] [CrossRef]
- Dasadia, A.K.; Nariya, B.B.; Jani, A.R. Growth and Structure Determination of ZrSTe—A New Ternary Phase of Transition Metal Chalcogenides. J. Cryst. Growth 2015, 426, 265–269. [Google Scholar] [CrossRef]
- Roubi, L.; Carlone, C. Resonance Raman Spectrum of HfS2 and ZrS2. Phys. Rev. B 1988, 37, 6808–6812. [Google Scholar] [CrossRef] [PubMed]
- Mañas-Valero, S.; García-López, V. Raman Spectra of ZrS2 and ZrSe2 from Bulk to Atomically Thin Layers. Appl. Sci. 2016, 6, 264. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Simpson, J.R.; Bertolazzi, S.; Brivio, J.; Watson, M.; Wu, X.; Kis, A.; Luo, T.; Hight Walker, A.R.; Xing, H.G. Thermal Conductivity of Monolayer Molybdenum Disulfide Obtained from Temperature-Dependent Raman Spectroscopy. ACS Nano 2014, 8, 986–993. [Google Scholar] [CrossRef]
- Ho, C.H.; Huang, Y.S.; Tiong, K.K.; Liao, P.C. Absorption-Edge Anisotropy in ReS2 and ReSe2 Layered Semiconductors. Phys. Rev. B 1998, 58, 16130–16135. [Google Scholar] [CrossRef]
- Ho, C.H.; Tseng, C.Y.; Tien, L.C. Thermoreflectance Characterization of β-Ga2O3 Thin-Film Nanostrips. Opt. Express 2010, 18, 16360–16369. [Google Scholar] [CrossRef]
- Ho, C.H.; Chen, H.H. Optically Decomposed Near-Band-Edge Structure and Excitonic Transitions in Ga2S3. Sci. Rep. 2014, 4, 6143. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.H.; Chan, C.H.; Tien, L.C.; Huang, Y.S. Direct Optical Observation of Band-Edge Excitons, Band Gap, and Fermi Level in Degenerate Semiconducting Oxide Nanowires In2O3. J. Phys. Chem. C 2011, 115, 25088–25096. [Google Scholar] [CrossRef]
- Ho, C.H.; Wang, Y.P.; Chan, C.H.; Huang, Y.S.; Li, C.H. Temperature-Dependent Photoconductivity in β-In2S3 Single Crystals. J. Appl. Phys. 2010, 108, 043518. [Google Scholar] [CrossRef]
- Moustafa, M.; Wasnick, A.; Janowitz, C.; Manzke, R. Temperature Shift of the Absorption Edge and Urbach Tail of ZrSxSe2−x Single Crystals. Phys. Rev. B 2017, 95, 245207. [Google Scholar] [CrossRef]
- Greenaway, D.L.; Nitsche, R. Preparation and Optical Properties of Group IV-VI2 Chalcogenides Having The CdI2 Structure. J. Phys. Chem. Solid. 1965, 26, 1445–1458. [Google Scholar] [CrossRef]
- Moustafa, M.; Zandt, T.; Janowitz, C.; Manzke, R. Growth and Band Gap Determination of the ZrSxSe2−x Single Cystal Series. Phys. Rev. B 2009, 80, 035206. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. In Two Dimensional Transition Metal Dichalcogenides. Mater. Sci. 2016, 239, 17–23. [Google Scholar]
- Ghafari, A.; Janowitz, C. Electronic and Thermoelectric Properties of ZrSxSe2−x. Comput. Mater. Sci. 2019, 169, 109109. [Google Scholar] [CrossRef]
- Yumnam, G.; Pandey, T.; Singh, A.K. High Temperature Thermoelectric Properties of Zr and Hf Based Transition Metal Dichalcogenides: A First Principles Study. J. Chem. Phys. 2015, 143, 234704. [Google Scholar] [CrossRef] [Green Version]
- Mleczko, M.J.; Zhang, C.; Lee, H.R.; Kuo, H.H.; Magyari-Köpe, B.; Moore, R.G.; Shen, Z.X.; Fisher, I.R.; Nishi, Y.; Pop, E. HfSe2 and ZrSe2: Two-Dimensional Semiconductors with Native High-k Oxides. Sci. Adv. 2017, 3, e1700481. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R. Thermoelectric Power Measurements of Zirconium Sulphoselenide Single Crystals. Int. J. Phys. Math. Sci. 2012, 2, 74–85. [Google Scholar]
- Guo, S.D.; Li, Y.F.; Guo, X.S. Predicted Janus Monolayer ZrSSe with Enhanced n-type Thermoelectric Properties Compared with Monolayer ZrS2. Comput. Mater. Sci. 2019, 161, 16–23. [Google Scholar] [CrossRef]
- Ho, C.H. The Study of Structure, Thermoelectric and Photoelectric Properties of Layered Tin Monochalcogenides SnX (X = S, Se) for Energy Application. ACS Appl. Energy Mater. 2020, 3. [Google Scholar] [CrossRef]
- Ding, G.; Gao, G.Y.; Huang, Z.; Zhang, W.; Yao, K. Thermoelectric Properties of Monolayer MSe2 (M = Zr, Hf): Low Lattice Thermal Conductivity and A Promising Figure of Merit. Nanotechnology 2016, 27, 375703. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herninda, T.M.; Ho, C.-H. Optical and Thermoelectric Properties of Surface-Oxidation Sensitive Layered Zirconium Dichalcogenides ZrS2−xSex (x = 0, 1, 2) Crystals Grown by Chemical Vapor Transport. Crystals 2020, 10, 327. https://doi.org/10.3390/cryst10040327
Herninda TM, Ho C-H. Optical and Thermoelectric Properties of Surface-Oxidation Sensitive Layered Zirconium Dichalcogenides ZrS2−xSex (x = 0, 1, 2) Crystals Grown by Chemical Vapor Transport. Crystals. 2020; 10(4):327. https://doi.org/10.3390/cryst10040327
Chicago/Turabian StyleHerninda, Thalita Maysha, and Ching-Hwa Ho. 2020. "Optical and Thermoelectric Properties of Surface-Oxidation Sensitive Layered Zirconium Dichalcogenides ZrS2−xSex (x = 0, 1, 2) Crystals Grown by Chemical Vapor Transport" Crystals 10, no. 4: 327. https://doi.org/10.3390/cryst10040327
APA StyleHerninda, T. M., & Ho, C.-H. (2020). Optical and Thermoelectric Properties of Surface-Oxidation Sensitive Layered Zirconium Dichalcogenides ZrS2−xSex (x = 0, 1, 2) Crystals Grown by Chemical Vapor Transport. Crystals, 10(4), 327. https://doi.org/10.3390/cryst10040327