A Complex Assemblage of Crystal Habits of Pyrite in the Volcanic Hot Springs from Kamchatka, Russia: Implications for the Mineral Signature of Life on Mars
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Single Crystals
3.2. Pyrite Crystal Aggregates
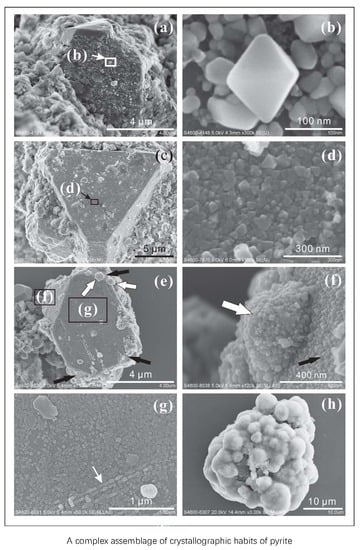
- yrite crystals (single crystals with their sizes ranging from 5 to 10 µm) forming aggregates of ~20 µm together with clay minerals (Figure 7a,b). The pyrite crystals in these aggregates have combinations of {100}, o{111}, and e{210} habits. This type of aggregate, with sizes ranging from 10 to 100 µm, was commonly found in the samples studied.
- Parallel intergrowths of pyrite nanocrystals (<300 nm) were observed, which attach to, or nucleate on the o{111} surface of larger pyrite crystals (>10 µm) (Figure 7c,d). The habits of these pyrite nanocrystals are mostly dominated by their o{111} form, which is sometimes slightly modified by e{210}.
- Pyrite crystal aggregates attaching to the other minerals (Figure 7e,f). They are tiled on the surfaces of the other larger crystals and commonly appear in irregular crystal morphologies. These larger minerals offer surfaces for small pyrite crystals to stick onto.
- Massive pyrite nanocrystals (<100 nm) were observed to attach to, or crystallize on the surface of large pyrite crystals (>5 µm) (Figure 8a–g). The habits of these nanocrystals are octahedral (Figure 8d), cubic (Figure 8g), and irregular (Figure 8b). Pyrite nanocrystals do not just attach to or overgrow some surfaces of larger crystals like type II, they also tile the surface. Different stages of pyrite nanocrystal development (irregular crystals with or without obscure edges) are shown in Figure 8b. Octahedral nanocrystals prefer to attach to o{111} faces while cubic ones prefer a{100} faces (Figure 8e–g). Some of them grow in a certain direction (Figure 8g).
- Irregular pyrite nanocrystals aggregate as spherulites (Figure 8e,f,h). Some of the small single aggregates (~500 nm) attach to the surfaces of large pyrite crystals (white arrows in Figure 8e,f). Some large aggregates (1–5 µm) attaching to other mineral surfaces are covered by thin films containing organic carbon and sulfur, as measured by EDS (Figure 8h).
3.3. Intergrowth Texture
- Intergrowth of single crystals. The cubical pyrite intergrowth texture was very common in the hot spring sediments. Cubical pyrite crystals with a size range of 5 to 10 μm show intergrowth with each other, which are sometimes coated by clay minerals (Figure 9a). The octahedral crystals ranging from 300 nm to 1 μm were observed to have intergrowth with each other (Figure 9b) and were covered by thin biofilms, as indicated by EDS measurements.
- Intergrowth of crystal combinations. Combination crystals of o{111} and a{100} show intergrowth with each other (Figure 9c). The individual crystals are around 3 μm in size. All faces of a{100} form are striated in a specific direction.
- Twin crystals appear as mirror images across the boundary where each crystal is combined with octahedron and cube habits (Figure 9e). The size of a whole crystal is about 3 μm. There are also other small pyrite crystals attached to the edges of twin crystals.
- Parallel growth with relative smooth a{100} forms and rough o{111} forms, which can be covered by a thin layer of clay minerals (Figure 9f). The dimension of a whole crystal is about 700 nm.
3.4. Spherulite Pyrite Crystals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boston, P.; Spilde, M.; Northup, D.; Melim, L.; Soroka, D.; Kleina, L.; Lavoie, K.; Hose, L.; Mallory, L.; Dahm, C. Cave biosignature suites: Microbes, minerals, and Mars. Astrobiology 2001, 1, 25–55. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.; Yang, H. Mineral biosignatures in evaporites: Presence of rosickyite in an endoevaporitic microbial community from Death Valley, California. Geology 2002, 30, 1075–1078. [Google Scholar] [CrossRef]
- Seager, S.; Turner, E.; Schafer, J.; Ford, E. Vegetation’s red edge: A possible spectroscopic biosignature of extraterrestrial plants. Astrobiology 2005, 5, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Fakra, S.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: Implications for biosignature formation. ISME J. 2011, 5, 717–727. [Google Scholar] [CrossRef]
- Summons, R.E.; Amend, J.P.; Bish, D.; Buick, R.; Cody, G.D.; Des Marais, D.J.; Dromart, G.; Eigenbrode, J.L.; Knoll, A.H.; Sumner, D.Y. Preservation of Martian organic and environmental records: Final report of the Mars Biosignature Working Group. Astrobiology 2011, 11, 157–181. [Google Scholar] [CrossRef]
- Banfield, J.F.; Moreau, J.W.; Chan, C.S.; Welch, S.A.; Little, B. Mineralogical biosignatures and the search for life on Mars. Astrobiology 2001, 1, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cady, S.L.; Farmer, J.D.; Grotzinger, J.P.; Schopf, J.W.; Steele, A. Morphological biosignatures and the search for life on Mars. Astrobiology 2003, 3, 351–368. [Google Scholar] [CrossRef]
- Dove, P.M.; De Yoreo, J.J.; Weiner, S. Biomineralization; Mineralogical Society of America and the Geochemical Society: Washington, DC, USA, 2003; Volume 54, pp. 10–17. [Google Scholar]
- Douglas, S. Microbial biosignatures in evaporite deposits: Evidence from Death Valley, California. Planet. Space Sci. 2004, 52, 223–227. [Google Scholar] [CrossRef]
- Fortin, D.; Langley, S. Formation and occurrence of biogenic iron-rich minerals. Earth Sci. Rev. 2005, 72, 1–19. [Google Scholar] [CrossRef]
- Golden, D.C.; Ming, D.W.; Morris, R.V.; Brearley, A.J.; Lauer, H.V.; Treiman, A.H.; Zolensky, M.E.; Schwandt, C.S.; Lofgren, G.E.; McKay, G.A. Evidence for exclusively inorganic formation of magnetite in Martian meteorite ALH84001. Am. Mineral. 2004, 89, 681–695. [Google Scholar] [CrossRef]
- Tang, M.; Ehreiser, A.; Li, Y.-L. Gypsum in modern Kamchatka volcanic hot springs and the Lower Cambrian black shale: Applied to the microbial-mediated precipitation of sulfates on Mars. Am. Mineral. 2014, 99, 2126–2137. [Google Scholar] [CrossRef] [Green Version]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Xian, H.; Zhu, J.; Liang, X.; He, H. Morphology controllable syntheses of micro- and nano-iron pyrite mono- and poly-crystals: A review. RSC Adv. 2016, 6, 31988–31999. [Google Scholar] [CrossRef]
- Luther, G.W., III. Pyrite synthesis via polysulfide compounds. Geochim. Cosmochim. Acta 1991, 55, 2839–2849. [Google Scholar] [CrossRef]
- Morse, J.W.; Wang, Q. Pyrite formation under conditions approximating those in anoxic sediments: II. Influence of precursor iron minerals and organic matter. Mar. Chem. 1997, 57, 187–193. [Google Scholar] [CrossRef]
- Rickard, D. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125 °C: The rate equation. Geochim. Cosmochim. Acta 1997, 61, 115–134. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W., III. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125 °C: The mechanism. Geochim. Cosmochim. Acta 1997, 61, 135–147. [Google Scholar] [CrossRef]
- Schoonen, M.A. Mechanisms of sedimentary pyrite formation. In Sulfur Biogeochemistry—Past and Present; Amend, J.P., Edwards, K.J., Lyons, T.W., Eds.; The Geological Society of America, Inc.: Boulder, CO, USA, 2004; pp. 117–134. [Google Scholar]
- Ohfuji, H.; Rickard, D. Experimental syntheses of framboids—A review. Earth Sci. Rev. 2005, 71, 147–170. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Shape controlled growth of pyrite FeS2 crystallites via a polymer-assisted hydrothermal route. CrystEngComm 2010, 12, 3797–3805. [Google Scholar] [CrossRef]
- Goldhaber, M.B. Sulfur-rich sediments. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; Volume 7, pp. 257–288. [Google Scholar]
- Rickard, D.; Luther, G.W., III. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Thiel, J.; Byrne, J.M.; Kappler, A.; Schink, B.; Pester, M. Pyrite formation from FeS and H2S is mediated by a novel type of microbial energy metabolism. bioRxiv 2018, 396978. [Google Scholar] [CrossRef] [Green Version]
- Fortin, D.; Beveridge, T.J. Microbial sulfate reduction within sulfidic mine tailings: Formation of diagenetic Fe sulfides. Geomicrobiol. J. 1997, 14, 1–21. [Google Scholar] [CrossRef]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Baeuerlein, E. Biomineralization: From Biology to Biotechnology and Medical Application; Wiley-VCH: Weinheim, Germany, 2004; pp. 159–176. [Google Scholar]
- Panda, A.K.; Bisht, S.S.; De Mandal, S.; Kumar, N.S. Bacterial and archeal community composition in hot springs from Indo-Burma region, North-east India. AMB Express 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berner, R.A. Sedimentary pyrite formation: An update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Severmann, S.; Mills, R.A.; Palmer, M.R.; Telling, J.P.; Cragg, B.; John Parkes, R. The role of prokaryotes in subsurface weathering of hydrothermal sediments: A combined geochemical and microbiological investigation. Geochim. Cosmochim. Acta 2006, 70, 1677–1694. [Google Scholar] [CrossRef]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. In Biomineralization; Dove, P.M., De Yoreo, J.J., Weiner, S., Eds.; Mineralogical Society of America and Geochemical Society: Washington, DC, USA, 2003; Volume 54, pp. 1–29. [Google Scholar]
- Sweeney, R.E.; Kaplan, I.R. Pyrite framboid formation: Laboratory synthesis and marine sediments. Econ. Geol. 1973, 68, 618–634. [Google Scholar] [CrossRef]
- Maclean, L.C.W.; Tyliszczak, T.; Gilbert, P.U.P.A.; Zhou, D.; Pray, T.J.; Onstott, T.C.; Southam, G. A high-resolution chemical and structural study of framboidal pyrite formed within a low-temperature bacterial biofilm. Geobiology 2008, 6, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Haymon, R.M.; Kastner, M. Hot spring deposits on the east pacific rise at 21° N: Preliminary description of mineralogy and genesis. Earth Planet. Sci. Lett. 1981, 53, 363–381. [Google Scholar] [CrossRef]
- Vargas, M.; Kashefi, K.; Blunt-Harris, E.L.; Lovley, D.R. Microbiological evidence for Fe(III) reduction on early Earth. Nature 1998, 395, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Westall, F. Early life on earth and analogies to mars. In Water on Mars and Life; Tokano, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 4, pp. 45–64. [Google Scholar]
- Yücel, M.; Gartman, A.; Chan, C.S.; Luther, G.W., III. Hydrothermal vents as a kinetically stable source of iron-sulphide-bearing nanoparticles to the ocean. Nat. Geosci. 2011, 4, 367–371. [Google Scholar] [CrossRef]
- Gartman, A.; Luther, G.W., III. Comparison of pyrite (FeS2) synthesis mechanisms to reproduce natural FeS2 nanoparticles found at hydrothermal vents. Geochim. Cosmochim. Acta 2013, 120, 447–458. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Canet, C.; Villanueva-Estrada, R.E.; Ortega-Osorio, A. Morphology of pyrite in particulate matter from shallow submarine hydrothermal vents. Am. Mineral. 2010, 95, 1500–1507. [Google Scholar] [CrossRef]
- Barghoorn, E.S.; Nichols, R.L. Sulfate-reducing bacteria and pyritic sediments in Antarctica. Science 1961, 134, 190. [Google Scholar] [CrossRef] [PubMed]
- Schieber, J. Sedimentary pyrite: A window into the microbial past. Geology 2002, 30, 531–534. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Senko, J.M.; Najar, F.Z.; Kenton, S.M.; Roe, B.A.; Dewers, T.A.; Spear, J.R.; Krumholz, L.R. Bacterial diversity and sulfur cycling in a mesophilic sulfide-rich spring. Appl. Environ. Microbiol. 2003, 69, 5609–5621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, I.D.; Wiegel, J. Diversity of thermophilic anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 1–43. [Google Scholar] [CrossRef]
- Rice, C.; Ashcroft, W.; Batten, D.; Boyce, A.; Caulfield, J.; Fallick, A.; Hole, M.; Jones, E.; Pearson, M.; Rogers, G. A Devonian auriferous hot spring system, Rhynie, Scotland. J. Geol. Soc. Lond. 1995, 152, 229–250. [Google Scholar] [CrossRef]
- Westall, F.; de Wit, M.J.; Dann, J.; van der Gaast, S.; de Ronde, C.E.J.; Gerneke, D. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 2001, 106, 93–116. [Google Scholar] [CrossRef]
- Westall, F. Morphological biosignatures in early terrestrial and extraterrestrial materials. In Strategies of Life Detection; Botta, O., Bada, J.L., Gomez-Elvira, J., Javaux, E., Selsis, F., Summons, R., Eds.; Springer: Boston, MA, USA, 2008; pp. 95–114. [Google Scholar]
- Bazylinski, D.; Frankel, R. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Chan, L.; Li, Y.-L. What lurks in the Martian rocks and soil? Investigations of sulfates, phosphates, and perchlorates. Flower-like apatite recording microbial processes through deep geological time and its implication to the search for mineral records of life on Mars. Am. Mineral. 2014, 99, 2116–2125. [Google Scholar] [CrossRef]
- Gorbatov, A.; Kostoglodov, V.; Suarez, G.; Gordeev, E. Seismicity and structure of the Kamchatka subduction zone. J. Geophys. Res. Solid Earth 1997, 102, 17883–17898. [Google Scholar] [CrossRef]
- Okrugin, V.M.; Tazaki, K.; Bel’kova, N.L. Hydrothermal mineral formation systems of Kamchatka and the biomineralization. In Proceedings: International Symposium of the Kanazawa University 21st-Century COE Program; Kamta, N., Ed.; Kanazawa University: Kanazawa, Japan, 2003; Volume 1, pp. 235–238. [Google Scholar]
- Kozhurin, A.; Acocella, V.; Kyle, P.R.; Lagmay, F.M.; Melekestsev, I.V.; Ponomareva, V.; Rust, D.; Tibaldi, A.; Tunesi, A.; Corazzato, C.; et al. Trenching studies of active faults in Kamchatka, eastern Russia: Palaeoseismic, tectonic and hazard implications. Tectonophysics 2006, 417, 285–304. [Google Scholar] [CrossRef]
- Karpov, G.A.; Naboko, S.I. Metal contents of recent thermal waters, mineral precipitates and hydrothermal alteration in active geothermal fields, Kamchatka. J. Geochem. Explor. 1990, 36, 57–71. [Google Scholar] [CrossRef]
- Waltham, T. A guide to the volcanoes of southern Kamchatka, Russia. Proc. Geol. Assoc. 2001, 112, 67–78. [Google Scholar] [CrossRef]
- Kontorovich, A.E.; Bortnikova, S.B.; Karpov, G.A.; Kashirtsev, V.A.; Kostyreva, E.A.; Fomin, A.N. Uzon volcano caldera (Kamchatka): A unique natural laboratory of the present-day naphthide genesis. Russ. Geol. Geophys. 2011, 52, 768–772. [Google Scholar] [CrossRef]
- Taran, Y. Geochemistry of volcanic and hydrothermal fluids and volatile budget of the Kamchatka–Kuril subduction zone. Geochim. Cosmochim. Acta 2009, 73, 1067–1094. [Google Scholar] [CrossRef]
- Zhao, W. Diversity and Potential Geochemical Functions of Prokaryotes in Hot Springs of the Uzon Caldera, Kamchatka. Ph.D. Thesis, The University of Georgia, Athens, GA, USA, 2008. [Google Scholar]
- Hollingsworth, E.R. Elemental and Isotopic Chemistry of the Uzon Caldera: The Evolution of Thermal Waters, Gas, and Mineral Precipitation. Master’s Thesis, The University of Georgia, Athens, GA, USA, 2006. [Google Scholar]
- Goin, J.C.; Cady, S.L. Biosedimentological processes that produce hot spring sinter biofabrics: Examples from the Uzon Caldera, Kamchatka Russia. In From Fossils to Astrobiology; Seckbach, J., Walsh, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 12, pp. 159–179. [Google Scholar]
- Kochetkova, T.; Rusanov, I.; Pimenov, N.; Kolganova, T.; Lebedinsky, A.; Bonch-Osmolovskaya, E.; Sokolova, T. Anaerobic transformation of carbon monoxide by microbial communities of Kamchatka hot springs. Extremophiles 2011, 15, 319–325. [Google Scholar] [CrossRef]
- Zhao, W.; Song, Z.; Jiang, H.; Li, W.; Mou, X.; Romanek, C.S.; Wiegel, J.; Dong, H.; Zhang, C.L. Ammonia-oxidizing Archaea in Kamchatka Hot Springs. Geomicrobiol. J. 2011, 28, 149–159. [Google Scholar] [CrossRef]
- Burgess, E.A. Geomicrobiological Description of Two Contemporary Hydrothermal Pools in Uzon Caldera, Kamchatka, Russia, as Models for Sulfur Biogeochemistry. Ph.D. Thesis, The University of Georgia, Athens, GA, USA, 2009. [Google Scholar]
- Burgess, E.; Unrine, J.; Mills, G.; Romanek, C.; Wiegel, J. Comparative geochemical and microbiological characterization of two thermal pools in the Uzon Caldera, Kamchatka, Russia. Microb. Ecol. 2012, 63, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Gumerov, V.M.; Mardanov, A.V.; Beletsky, A.V.; Prokofeva, M.I.; Bonch-Osmolovskaya, E.A.; Ravin, N.V.; Skryabin, K.G. Complete genome sequence of “Vulcanisaeta moutnovskia” strain 768-28, a novel member of the hyperthermophilic crenarchaeal genus Vulcanisaeta. J. Bacteriol. 2011, 193, 2355–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, J.E. Mineral-Microbe Interactions and Biomineralization of Siliceous Sinters and Underlying Rock from Jenn’s Pools in the Uzon Caldera, Kamchatka, Russia. Master’s Thesis, The University of Georgia, Athens, GA, USA, 2005. [Google Scholar]
- Kyle, J.E.; Schroeder, P.A.; Wiegel, J. Microbial silicification in sinters from two terrestrial hot springs in the Uzon Caldera, Kamchatka, Russia. Geomicrobiol. J. 2007, 24, 627–641. [Google Scholar] [CrossRef]
- McCammon, C. Mössbauer spectroscopy of minerals. In Mineral Physics and Crystallography: A Handbook of Physical Constants; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; Volume 2, pp. 332–347. [Google Scholar]
- Evans, B.J.; Johnson, R.G.; Senftle, F.E.; Cecil, C.B.; Dulong, F. The 57Fe Mössbauer parameters of pyrite and marcasite with different provenances. Geochim. Cosmochim. Acta 1982, 46, 761–775. [Google Scholar] [CrossRef] [Green Version]
- Dyar, M.D.; Agresti, D.G.; Schaefer, M.W.; Grant, C.A.; Sklute, E.C. Mössbauer spectroscopy of earth and planetary materials. Annu. Rev. Earth Planet. Sci. 2006, 34, 83–125. [Google Scholar] [CrossRef] [Green Version]
- Bethke, C.M.; Yeakel, S. The Geochemist’s Workbench, Release 7.0: GWB Essentials Guide; Hydrogeology Program; University of Illinois: Urbana, IL, USA, 2007; pp. 1–98. [Google Scholar]
- Kublanov, I.V.; Perevalova, A.A.; Slobodkina, G.B.; Lebedinsky, A.V.; Bidzhieva, S.K.; Kolganova, T.V.; Kaliberda, E.N.; Rumsh, L.D.; Haertlé, T.; Bonch-Osmolovskaya, E.A. Biodiversity of thermophilic prokaryotes with hydrolytic activities in hot springs of Uzon Caldera, Kamchatka (Russia). Appl. Environ. Microbiol. 2009, 75, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Mardanov, A.V.; Gumerov, V.M.; Beletsky, A.V.; Perevalova, A.A.; Karpov, G.A.; Bonch-Osmolovskaya, E.A.; Ravin, N.V. Uncultured archaea dominate in the thermal groundwater of Uzon Caldera, Kamchatka. Extremophiles 2011, 15, 365–372. [Google Scholar] [CrossRef]
- Slobodkina, G.B.; Panteleeva, A.N.; Sokolova, T.G.; Bonch-Osmolovskaya, E.A.; Slobodkin, A.I. Carboxydocella manganica sp nov., a thermophilic, dissimilatory Mn(IV)- and Fe(III)-reducing bacterium from a Kamchatka hot spring. Int. J. Syst. Evol. Microbiol. 2012, 62, 890–894. [Google Scholar] [CrossRef] [Green Version]
- Wilkin, R.T.; Barnes, H.L. Pyrite formation in an anoxic estuarine basin. Am. J. Sci. 1997, 297, 620–650. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, CA, USA, 2005; p. 4129. [Google Scholar]
- Blackburn, W.H.; Dennen, W.H. Principles of Mineralogy; William C Brown Pub: Dubuque, IA, USA, 1994; p. 413. [Google Scholar]
- Steiner, M.; Wallis, E.; Erdtmann, B.-D.; Zhao, Y.; Yang, R. Submarine-hydrothermal exhalative ore layers in black shales from South China and associated fossils—Insights into a lower Cambrian facies and bio-evolution. Palaeogeogr. Palaeoclim. Palaeoecol. 2001, 169, 165–191. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Chen, Y.-Q.; Ling, H.-F.; Yang, J.-H.; Feng, H.-Z.; Ni, P. Trace- and rare-earth element geochemistry and Pb–Pb dating of black shales and intercalated Ni–Mo–PGE–Au sulfide ores in Lower Cambrian strata, Yangtze Platform, South China. Min. Depos. 2006, 41, 453–467. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.-L. An SEM study of microfossils in the black shale of the lower Cambrian niutitang formation, southwest China: Implications for the polymetallic sulfide mineralization. Ore Geol. Rev. 2015, 65, 811–820. [Google Scholar] [CrossRef]
- Juniper, S.K.; Thompson, J.A.J.; Calvert, S.E. Accumulation of minerals and trace elements in biogenic mucus at hydrothermal vents. Deep Sea Res. Part A Oceanogr. Res. Pap. 1986, 33, 339–347. [Google Scholar] [CrossRef]
- Thomas-Keprta, K.L.; Bazylinski, D.A.; Kirschvink, J.L.; Clemett, S.J.; McKay, D.S.; Wentworth, S.J.; Vali, H.; Gibson, E.K., Jr.; Romanek, C.S. Elongated prismatic magnetite crystals in ALH84001 carbonate globules: Potential Martian magnetofossils. Geochim. Cosmochim. Acta 2000, 64, 4049–4081. [Google Scholar] [CrossRef]
- Thomas-Keprta, K.L.; Clemett, S.J.; Bazylinski, D.A.; Kirschvink, J.L.; McKay, D.S.; Wentworth, S.J.; Vali, H.; Gibson, E.K.; McKay, M.F.; Romanek, C.S. Truncated hexa-octahedral magnetite crystals in ALH84001: Presumptive biosignatures. Proc. Natl. Acad. Sci. USA 2001, 98, 2164–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas-Keprta, K.L.; Clemett, S.J.; Bazylinski, D.A.; Kirschvink, J.L.; McKay, D.S.; Wentworth, S.J.; Vali, H.; Gibson, E.K., Jr.; Romanek, C.S. Magnetofossils from ancient Mars: A robust biosignature in the Martian meteorite ALH84001. Appl. Environ. Microbiol. 2002, 68, 3663–3672. [Google Scholar] [CrossRef] [Green Version]
- Fisk, M.R.; Giovannoni, S.J.; Thorseth, I.H. Alteration of oceanic volcanic glass: Textural evidence of microbial activity. Science 1998, 281, 978–980. [Google Scholar] [CrossRef]
- MacLachlan, M.; Manners, I.; Ozin, G.A. New (inter)faces: Polymers and inorganic materials. Adv. Mater. 2000, 12, 675–681. [Google Scholar] [CrossRef]
- Barbieri, R.; Stivaletta, N.; Marinangeli, L.; Ori, G.-G. Microbial signatures in sabkha evaporite deposits of Chott el Gharsa (Tunisia) and their astrobiological implications. Planet. Space Sci. 2006, 54, 726–736. [Google Scholar] [CrossRef]
- Hofmann, B.; Farmer, J. Filamentous fabrics in low-temperature mineral assemblages: Are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet. Space Sci. 2000, 48, 1077–1086. [Google Scholar] [CrossRef]
- Zolotov, M.Y.; Shock, E.L. Formation of jarosite-bearing deposits through aqueous oxidation of pyrite at meridiani planum, mars. Geophys. Res. Lett. 2005, 32, L21203. [Google Scholar] [CrossRef] [Green Version]
- Vaniman, D.T.; Bish, D.L.; Ming, D.W.; Bristow, T.F.; Morris, R.V.; Blake, D.F.; Chipera, S.J.; Morrison, S.M.; Treiman, A.H.; Rampe, E.B.; et al. Mineralogy of a mudstone at Yellowknife Bay, Gale crater, Mars. Science 2014, 343, 1243480. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.P.; Mojzsis, S.J.; Coath, C.D. Sulfur isotopic compositions of individual sulfides in Martian meteorites ALH84001 and Nakhla: Implications for crust–regolith exchange on Mars. Earth Planet. Sci. Lett. 2000, 184, 23–35. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Pont, S.; Chevrier, V.; Luguet, A.; Zanda, B.; Hewins, R. Petrogenesis of Martian sulfides in the chassigny meteorite. Am. Mineral. 2018, 103, 872–885. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Hewins, R.; Remusat, L.; Zanda, B.; Pont, S.; Leroux, H.; Marinova, M.; Jacob, D.; Humayun, M.; Nemchin, A.; et al. Nickeliferous pyrite tracks pervasive hydrothermal alteration in Martian regolith breccia: A study in nwa 7533. Meteorit. Planet. Sci. 2015, 50, 2099–2120. [Google Scholar] [CrossRef]
- Wittmann, A.; Korotev, R.L.; Jolliff, B.L.; Irving, A.J.; Moser, D.E.; Barker, I.; Rumble, D., III. Petrography and composition of Martian regolith breccia meteorite northwest Africa 7475. Meteorit. Planet. Sci. 2015, 50, 326–352. [Google Scholar] [CrossRef]
- Boston, P.; Ivanov, M.; McKay, C. On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus 1992, 95, 300–308. [Google Scholar] [CrossRef]
- Allen, C.C.; Oehler, D.Z. A case for ancient springs in Arabia Terra, Mars. Astrobiology 2008, 8, 1093–1112. [Google Scholar] [CrossRef]










| Parameters | Burlyashii | Zavarzin | Thermophile | Jen’s Vent 1 | Jen’s Vent 2 |
|---|---|---|---|---|---|
| Temperature (°C) | 51–87 | 54–74 | 42–70 | 83 | 85 |
| Eh (mV) | −90 | −96 | −240 | −240 | |
| pH | 6–6.5 | 5.5–7.5 | 4.4–7 | 5.3–5.9 | 5.3–5.9 |
| Alkalinity | 1.18–1.23 | 2.2 | 0.16–0.18 | ||
| Soluble Fe | 3.75 × 10−3 | 0.18 × 10−3 | 0.54 × 10−3 | ||
| SO42− | 0.23–2.3 | 0.335–0.557 | 0.1–0.3 | 1.35–1.96 | 1.29–3.125 |
| S2− | (6.3–43.8) × 10−3 | (0.6–43.1) × 10−3 | |||
| NO3− | 0.5 | 0.063 | 0.011 | ||
| NO2− | (0.1–0.3) × 10−3 | (0.2–0.6) × 10−3 | 0.41 × 10−3 | 0.54 × 10−3 | |
| NH4+ | 1.1–1.5 | 0.84 | 0.2–4 | ||
| References | [58,59,60] | [57,58,59,61,62,63] | [57,58,60] | [64,65] | [64,65] |
| Sample | Mineral | ISa (mm/s) | QSb (mm/s) | Area (%) |
|---|---|---|---|---|
| Jen’s Vent 1 | FeS2 | 0.31 | 0.55 | 56.66 |
| Silicate | 1.06 | 2.13 | 43.34 | |
| Oil Pool | FeS2 | 0.30 | 0.60 | 96.65 |
| Silicate | 1.22 | 1.99 | 3.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Li, Y.-L. A Complex Assemblage of Crystal Habits of Pyrite in the Volcanic Hot Springs from Kamchatka, Russia: Implications for the Mineral Signature of Life on Mars. Crystals 2020, 10, 535. https://doi.org/10.3390/cryst10060535
Tang M, Li Y-L. A Complex Assemblage of Crystal Habits of Pyrite in the Volcanic Hot Springs from Kamchatka, Russia: Implications for the Mineral Signature of Life on Mars. Crystals. 2020; 10(6):535. https://doi.org/10.3390/cryst10060535
Chicago/Turabian StyleTang, Min, and Yi-Liang Li. 2020. "A Complex Assemblage of Crystal Habits of Pyrite in the Volcanic Hot Springs from Kamchatka, Russia: Implications for the Mineral Signature of Life on Mars" Crystals 10, no. 6: 535. https://doi.org/10.3390/cryst10060535
APA StyleTang, M., & Li, Y. -L. (2020). A Complex Assemblage of Crystal Habits of Pyrite in the Volcanic Hot Springs from Kamchatka, Russia: Implications for the Mineral Signature of Life on Mars. Crystals, 10(6), 535. https://doi.org/10.3390/cryst10060535