Design, Synthesis, Crystal Structure, and Fungicidal Activity of Two Fenclorim Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthetic Procedure
2.2.1. Synthesis of (Z)-methyl 2-iodo-3-methoxyacrylate (2)
2.2.2. (E)-Methyl-3-methoxy-2-(2-phenoxyphenyl)acrylate (3)
2.2.3. (E)-Methyl-3-methoxy-2-(2-hydroxyphenyl)acrylate (4)
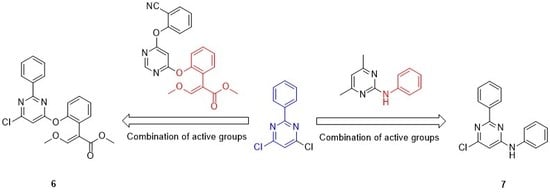
2.2.4. Methyl (E)-2-{2-[(6-chloro-2-phenylpyrimidin-4-yl)oxy]phenyl}-3-methoxyacrylate (6)
2.2.5. 6-chloro-N-2-diphenylpyrimidin-4-amine (7)
2.3. Structural Determination
2.4. Fungicidal Activity
3. Results and Discussion
3.1. Synthesis and Spectroscopic Properties
3.2. Crystal Structures of Compounds 6 and 7
3.3. Fungicidal Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mardanova, A.; Lutfullin, M.; Hadieva, G.; Akosah, Y.; Pudova, D.; Kabanov, D.; Shagimardanova, E.; Vankov, P.; Vologin, S.; Gogoleva, N.; et al. Structure and variation of root-associated microbiomes of potato grown in alfisol. World J. Microbiol. Biotechnol. 2019, 35, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Xia, D.G.; Hu, R.; Wang, W.; Cheng, X.; Wang, A.L.; Zhang, Q.; Lv, X.H. A bioactivity-oriented modification strategy for SDH inhibitors with superior activity against fungal strains. Pestic. Biochem. Physiol. 2020, 163, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Saklani, B.K.; Singh, P.K.; Asthana, R.K.; Sharma, T.R. Variation in the LRR region of Pi54 protein alters its interaction with the AvrPi54 protein revealed by in silico analysis. PLoS ONE 2019, 14, e0224088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, W.D.; de Souza Silva, J.V.; Guimaraes, C.M.; Sousa Ferreira, J.L.; Turozi, T.A.; Colodel, S. Use of copper-based pesticides to control fungal diseases of soybean in Northern Brazil. J. Exp. Agric. Int. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, P.; Sun, T.; Jin, X.; Yang, X.; Ling, Y. Design, Synthesis, and fungicidal activity of novel thiosemicarbazide derivatives containing piperidine fragments. Molecules 2017, 22, 2085. [Google Scholar] [CrossRef] [Green Version]
- Da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Yoshimoto, Y.; Arimori, S.; Kiguchi, S.; Harada, T.; Iwahashi, F. Discovery of metyltetraprole: Identification of tetrazolinone pharmacophore to overcome QoI resistance. Bioorg. Med. Chem. 2020, 28, 115211. [Google Scholar] [CrossRef]
- Odilbekov, F.; Edin, E.; Mostafanezhad, H.; Coolman, H.; Grenville-Briggs, L.J.; Liljeroth, E. Within-season changes in Alternaria solani populations in potato in response to fungicide application strategies. Eur. J. Plant Pathol. 2019, 155, 953–965. [Google Scholar] [CrossRef] [Green Version]
- Vaghefi, N.; Hay, F.S.; Kikkert, J.R.; Pethybridge, S.J. Genotypic diversity and resistance to azoxystrobin of Cercospora beticola on processing table beet in New York. Plant Dis. 2016, 100, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Li, M. Transcriptional profiling of ESTs from the biocontrol fungus Chaetomium cupreum. Sci. World J. 2012, 1–7. [Google Scholar]
- Zhao, J.; Bi, Q.; Wu, J.; Lu, F.; Han, X.; Wang, W. Occurrence and management of fungicide resistance in Botrytis cinerea on tomato from greenhouses in Hebei, China. J. Phytopathol. 2019, 167, 413–421. [Google Scholar] [CrossRef]
- Zheng, W.N.; Zhu, Z.Y.; Deng, Y.N.; Wu, Z.C.; Zhou, Y.; Zhou, X.M.; Bai, L.Y.; Deng, X.L. Synthesis, Crystal structure, herbicide safening, and antifungal activity of N-(4,6-dichloropyrimidine-2-yl) benzamide. Crystals 2018, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.L.; Zheng, W.N.; Zhou, X.M.; Bai, L.Y. The effect of salicylic acid and 20 substituted molecules on alleviating metolachlor herbicide injury in rice (Oryza sativa). Agronomy 2020, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Swiecilo, A.; Krzepilko, A.; Michalek, S. Evaluation of azoxystrobin toxicity to saprophytic fungi and radish in the early stages of growth. Ecol. Chem. Eng. A 2018, 25, 81–92. [Google Scholar]
- Berry, E.A.; Huang, L.S. Conformationally linked interaction in the cytochrome bc1 complex between inhibitors of the Qo site and the Rieske iron-sulfur protein. Biochim. Biophys. Acta-Bioenerg. 2011, 1807, 1349–1363. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Tang, T.; Tang, G.; Liang, Y.; Wang, W.C.; Yang, J.L.; Niu, J.F.; Tang, J.Y.; Zhou, Z.Y.; Cao, Y.S. Pyrimethanil ionic liquids paired with various natural organic acid anions for reducing its adverse impacts on the environment. J. Agric. Food Chem. 2019, 67, 11018–11024. [Google Scholar] [CrossRef]
- Milling, R.J.; Richardson, C.J. Mode of action of the anilino-pyrimidine fungicide pyrimethanil. 2. Effects on enzyme secretion in Botrytis cinerea. Pestic. Sci. 1995, 45, 43–48. [Google Scholar] [CrossRef]
- Miao, H.J.; Zhang, J.W.; Yuan, H.Z.; Li, Y.; Xu, Y.; Li, H.; Yang, X.L.; Ling, Y. Synthesis and fungicidal activities of nucleoside compounds containing substituted benzoyl thiourea. Chin. J. Org. Chem. 2012, 32, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhou, Y. Design, synthesis and insecticidal activity of some novel diacylhydrazine and acylhydrazone derivatives. Molecules 2015, 20, 5625–5637. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Song, H.; Liu, W.; Xu, C. Design, synthesis and antifungal activity of novel thioureas containing 1,3,4-thiadiazole and thioether skeleton. Chem. Res. Chin. Univ. 2016, 32, 615–620. [Google Scholar] [CrossRef]
- Liu, Y.G.; Luo, Y.; Lu, Y. A concise synthesis of azoxystrobin using a Suzuki cross-coupling reaction. J. Chem. Res. 2015, 39, 586–589. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, Y.B.; Chen, Z.B.; Wang, Y.L. Synthesis and anticoccidial activities of quinoline carboxylate derivatives with methyl (E)-2-(3-methoxy) acrylate moiety. Asian. J. Chem. 2013, 25, 8509–8512. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Xue, C. Synthesis of pyrimethanil. Pestic. Sci. Admin. 2005, 26, 24–25. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Li, H.C.; Guan, A.Y.; Huang, G.; Liu, C.L.; Li, Z.N.; Xie, Y.; Lan, J. Design, synthesis and structure-activity relationship of novel diphenylamine derivatives. Bioorg. Med. Chem. 2016, 24, 453–461. [Google Scholar] [CrossRef]
- Guan, A.; Liu, C.; Chen, W.; Yang, F.; Xie, Y.; Zhang, J.; Li, Z.; Wang, M. Design, synthesis, and structure-activity relationship of new pyrimidinamine derivatives containing an aryloxy pyridine moiety. J. Agric. Food Chem. 2017, 65, 1272–1280. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jia, G.K.; Yuan, L.; Bai, P.F.; He, H.; Zhou, Q. Syntheses, crystal structures and biological activities of three new Schiff bases derived from substituted salicylaldehyde and tris base. Chin. J. Struct. Chem. 2017, 36, 1797–1802. [Google Scholar]
- Mahgoub, M.Y.; Elmaghraby, A.M.; Harb, A.E.A.; Mahgoub, M.Y.; Ferreira, D.S.J.L.; Justino, G.C.; Marques, M.M. Synthesis, crystal structure, and biological evaluation of fused thiazolo [3,2-a] pyrimidines as new acetylcholinesterase inhibitors. Molecules 2019, 24, 2306. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.L.; Zhou, X.M.; Wang, Z.Y.; Rui, C.H.; Yang, X.L. Synthesis, crystal structure and insecticidal activity of N-(pyridin-2-ylmethyl)-1-phenyl-1,4,5,6,7,8-hexahydrocyclohepta[c]pyrazole-3-carbox amide. Chin. J. Struct. Chem. 2018, 37, 551–556. [Google Scholar]
- Shi, J.T.; Gong, Y.L.; Li, J.; Wang, Y.; Chen, Y.; Ding, S.; Liu, J. Synthesis, structure and biological activity of 2-[2-(4-fluorobenzylidene)hydrazinyl]-4-(1-methyl-1H-indol-3-yl)thieno[3,2-d]pyrimidine. Chin. J. Struct. Chem. 2019, 38, 1530–1536. [Google Scholar]
- Tao, Y.; Han, L.; Sun, A.; Sun, K.; Zhang, Q.; Liu, W.; Du, J.; Liu, Z. Crystal structure and computational study on methyl-3-aminothiophene-2-carboxylate. Crystals 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Manchado, A.; Salgado, M.M.; Vicente, A.; Diez, D.; Sanz, F.; Garrido, N.M. Crystal structure of methyl (4R)-4-(4-meth-oxy-benzo-yl)-4-{(1R)-1-phenyl-eth-ylcarbamo-yl}butanoate. Acta Crystallogr. Sect. E 2017, 73, 503–506. [Google Scholar] [CrossRef]
- Shen, Z.H.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhao, W.G. Synthesis, crystal structure, DFT studies and biological activity of a novel schiff base containing triazolo 4,3-a pyridine moiety. Chin. J. Struct. Chem. 2016, 35, 457–464. [Google Scholar]
- Herschlag, D.; Pinney, M.M. Hydrogen bonds: Simple after all? Biochemistry 2018, 57, 3338–3352. [Google Scholar] [CrossRef] [Green Version]






| Compound | 6 | 7 |
|---|---|---|
| CCDC No. | 1878381 | 1870401 |
| Empirical formula | C21H17ClN2O4 | C16H12ClN3 |
| Formula weight | 396.82 | 281.74 |
| Temperature/K | 100.00(10) | 100.00(10) |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | Cc |
| a/Å | 8.4842(6) | 10.2347(7) |
| b/Å | 24.457(2) | 18.3224(10) |
| c/Å | 8.9940(6) | 7.2447(4) |
| α/° | 90 | 90 |
| β/° | 96.305(6) | 92.266(6) |
| γ/° | 90 | 90 |
| Volume/Å3 | 1855.0(2) | 1357.50(14) |
| Z | 4 | 4 |
| ρcalcg/cm3 | 1.421 | 1.379 |
| μ/mm−1 | 2.092 | 2.418 |
| F(000) | 824.0 | 584.0 |
| Crystal size/mm3 | 0.11 × 0.11 × 0.08 | 0.14 × 0.13 × 0.12 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2Θ range for data collection/° | 7.228 to 177.332 | 9.654 to 148.926 |
| Index ranges | −10 ≤ h ≤ 10, −29 ≤ k ≤ 30, −8 ≤ l ≤ 10 | −12 ≤ h ≤ 8, −22 ≤ k ≤ 21, −8 ≤ l ≤ 8 |
| Reflections collected | 6427 | 4819 |
| Independent reflections | 3608 [Rint = 0.1240, Rsigma = 0.1384] | 1795 [Rint = 0.0317, Rsigma = 0.0245] |
| Data/restraints/parameters | 3608/54/255 | 1795/2/181 |
| Goodness-of-fit on F2 | 1.053 | 1.053 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0697, wR2 = 0.1650 | R1 = 0.0372, wR2 = 0.0985 |
| Final R indexes [all data] | R1 = 0.0835, wR2 = 0.1869 | R1 = 0.0376, wR2 = 0.0990 |
| Largest diff. peak/hole/ e Å−3 | 1.17/−1.33 | 0.22/−0.30 |
| Bond | Distance (Å) | Bond | Distance (Å) |
|---|---|---|---|
| C(12)–O(1) | 1.338(5) | C(1)–O(1) | 1.362(5) |
| C(10)–O(2) | 1.386(6) | C(15)–N(1) | 1.338(5) |
| C(11)–O(2) | 1.472(6) | C(15)−N(2) | 1.308(6) |
| C(8)–O(4) | 1.353(6) | C(14)−N(2) | 1.396(6) |
| C(9)–O(4) | 1.537(6) | C(8)−O(3) | 1.229(6) |
| C(12)–N(1) | 1.412(5) | C(14)−Cl(1) | 1.692(5) |
| C(15)−C(16) | 1.556(5) | C(14)−C(13) | 1.367(6) |
| C(1)−C(6) | 1.397(6) | C(5)−C(4) | 1.380(6) |
| C(12)−C(13) | 1.362(6) | C(20)−C(19) | 1.371(6) |
| C(15)−C(16) | 1.556(5) | C(7)−C(10) | 1.391(6) |
| Angle | (°) | Angle | (°) |
| C(12)−O(1)−C(1) | 115.3(3) | O(1)−C(12)−C13 | 112.2(4) |
| C(10)−O(2)−C(11) | 116.7(4) | N(1)−C(15)−C16 | 118.7(4) |
| C(8)−O(4)−C(9) | 118.7(4) | N(2)−C(15)−N1 | 121.5(4) |
| C(15)−N(1)−C(12) | 117.7(4) | N(2)−C(15)−C16 | 119.8(3) |
| C(15)−N(2)−C(14) | 118.2(4) | O(1)−C(1)−C2 | 115.0(4) |
| O(1)−C(12)−N(1) | 122.2(4) | O(1)−C(1)−C(6) | 119.9(4) |
| N(2)−C(14)−C(11) | 118.7(3) | C(13)−C(14)−N(2) | 126.4(4) |
| O(4)−C(8)−C(7) | 115.5(3) | O(3)−C(8)−O(4) | 118.3(4) |
| Bond | Distance (Å) | Bond | Distance (Å) |
|---|---|---|---|
| Cl(1)–C(8) | 1.732(3) | C(1)−C(2) | 1.399(4) |
| N(3)−C(11) | 1.413(4) | C(1)−C(6) | 1.401(4) |
| N(3)−C(10) | 1.356(4) | C(5)−C(6) | 1.384(4) |
| N(1)−C(7) | 1.343(4) | C(5)−C(4) | 1.389(4) |
| N(1)−C(8) | 1.339(4) | C(9)−C(8) | 1.360(4) |
| N(2)−C(7) | 1.337(3) | C(9)−C(10) | 1.412(4) |
| N(2)−C(10) | 1.341(4) | C(15)−C(14) | 1.390(4) |
| C(11)−C(16) | 1.393(4) | C(2)−C(3) | 1.383(4) |
| C(11)−C(12) | 1.395(4) | C(3)−C(4) | 1.395(4) |
| C(1)−C(7) | 1.482(4) | C(14)−C(13) | 1.383(5) |
| Angle | (°) | Angle | (°) |
| C(10)−N(3)−C(11) | 126.5(2) | N(2)−C(7)−C(1) | 117.3(2) |
| C(8)−N(1)–C(7) | 114.6(2) | N(1)−C(8)−Cl(1) | 114.9(2) |
| C(7)−N(2)−C(10) | 117.3(2) | N(1)−C(8)−C(9) | 125.2(3) |
| C(16)−C(11)−N(3) | 118.5(2) | C(9)−C(8)−Cl(1) | 119.9(2) |
| C(16)−C(11)−C(12) | 119.6(3) | N(3)−C(10)−C(9) | 119.5(2) |
| C(12)−C(11)−N(3) | 121.9(2) | N(2)−C(10)−N(3) | 119.4(2) |
| C(15)−C(16)−C(11) | 120.3(3) | N(2)−C(10)−C(9) | 121.1(2) |
| C(2)−C(1)−C(7) | 120.7(2) | C(3)−C(2)−C(1) | 120.7(3) |
| C(2)−C(1)−C(6) | 118.6(3) | C(2)−C(3)−C(4) | 120.2(3) |
| C(6)−C(1)−C(7) | 120.7(2) | C(5)−C(6)−C1) | 120.6(3) |
| D–H···A | d(D–H)/(Å) | d(H···A)/(Å) | d(D···A)/(Å) | <(DHA)/(°) |
|---|---|---|---|---|
| C(5)–H(5)···N(2) | 0.93 | 2.53 | 3.341(6) | 146 |
| C(11)–H(11B)···O(3) | 0.96 | 2.30 | 3.162(7) | 148 |
| D–H···A | d(D–H)/(Å) | d(H···A)/(Å) | d(D···A)/(Å) | <(DHA)/(°) |
|---|---|---|---|---|
| N(3)–H(3)···N(1) | 0.86 | 2.44 | 3.190(3) | 146 |
| C(2)–H(2)···N(1) | 0.93 | 2.48 | 2.802(4) | 100 |
| C(6)–H(6)···N(2) | 0.93 | 2.50 | 2.817(4) | 100 |
| C(12)–H(12)···N(2) | 0.93 | 2.57 | 2.930(4) | 104 |
| Comp. | EC50 (± SD) (mg/L) | Comp. | EC50 (± SD) (mg/L) |
|---|---|---|---|
| 6 | 20.84 ± 4.04 | 7 | 215.45 ± 55.43 |
| fenclorim | 319.95 ± 30.62 | pyrimethanil | 30.72 ± 3.78 |
| Compounds | Dose (mg/L) | P. Cubensis | E. Cichoracearum | B. Graminis | R. Solani | P. Polysora |
|---|---|---|---|---|---|---|
| Inhibitory Rate (%) | ||||||
| 6 | 200 | 94.00 ± 1.73 | 1.67 ± 2.88 | 40.67 ± 1.15 | 91.67 ± 2.89 | 50.00 ± 0 |
| 50 | 90.00 ± 2.00 | 0 | 41.33 ± 1.15 | 59.33 ± 1.15 | 8.33 ± 2.89 | |
| 12.5 | 80.33 ± 1.53 | 0 | 27.00 ± 1.73 | 58.33 ± 2.89 | 0 | |
| 7 | 200 | 93.33 ± 2.89 | 91.00 ± 3.61 | 90.67 ± 1.15 | 70.67 ± 1.15 | 90.67 ± 2.31 |
| 50 | 71.67 ± 2.89 | 89.00 ± 3.61 | 59.33 ± 1.15 | 11.67 ± 2.89 | 58.33 ± 2.89 | |
| 12.5 | 26.67 ± 2.89 | 31.67 ± 2.89 | 14.00 ± 1.73 | 7.67 ± 2.52 | 0 | |
| Fenclorim | 200 | 92.33 ± 2.52 | 0 | 23.33 ± 2.89 | 26.67 ± 2.89 | 38.33 ± 2.89 |
| 50 | 85.67 ± 2.08 | 0 | 22.33 ± 2.52 | 28.33 ± 2.89 | 21.67 ± 1.89 | |
| 12.5 | 22.33 ± 2.52 | 0 | 0 | 6.67 ± 2.89 | 0 | |
| Pyrimethanil | 200 | 89.00 ± 3.61 | 31.00 ± 3.61 | 28.33 ± 2.89 | 10 ± 0 | 31.33 ± 1.15 |
| 50 | 80.67 ± 1.15 | 6.00 ± 1.73 | 26.00 ± 1.73 | 9.33 ± 1.15 | 9.33 ± 1.15 | |
| 12.5 | 20.67 ± 1.15 | 0 | 19.33 ± 1.15 | 0 | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, K.-J.; Du, F.-P. Design, Synthesis, Crystal Structure, and Fungicidal Activity of Two Fenclorim Derivatives. Crystals 2020, 10, 587. https://doi.org/10.3390/cryst10070587
Xiong K-J, Du F-P. Design, Synthesis, Crystal Structure, and Fungicidal Activity of Two Fenclorim Derivatives. Crystals. 2020; 10(7):587. https://doi.org/10.3390/cryst10070587
Chicago/Turabian StyleXiong, Ke-Jie, and Feng-Pei Du. 2020. "Design, Synthesis, Crystal Structure, and Fungicidal Activity of Two Fenclorim Derivatives" Crystals 10, no. 7: 587. https://doi.org/10.3390/cryst10070587
APA StyleXiong, K. -J., & Du, F. -P. (2020). Design, Synthesis, Crystal Structure, and Fungicidal Activity of Two Fenclorim Derivatives. Crystals, 10(7), 587. https://doi.org/10.3390/cryst10070587