Reactive blends of PLA and TPCS compatibilized with MA using L101 as peroxide initiator were produced and characterized. GRH nanoplatelets were introduced with the objective of increasing the toughening of the reactive blends. Subsequently, cast films were produced and characterized. PLA-c and PLA-GRH were produced as reference films, and their properties are also reported.
3.1. Molecular Weight
The
Mn,
Mw, and
PI values of the PLA films are reported in
Table 3. The
Mn and
Mw for the fraction of PLA measured decreased significantly for the reactive blend of PLA-
g-TPCS with respect to PLA-c. The overall reduction of the PLA-
g-TPCS is related to several factors: (1) the use of MA and the peroxide initiator; (2) the presence of water and glycerol on TPCS; (3) the use of a two-step processing method (twin-screw extruder for the production of blends and single extruder for the production of films); and (4) the thermal conditions associated with the process.
The degradation of PLA in the reactive blends can be associated with the dominant side reaction occurring in the maleated PLA obtained using a free radical grafting initiator, such as L101, and a compatibilizer, such as MA [
19].
The presence of water due to the incorporation of TPCS for the production of the reactive blend is a reasonable factor affecting the reduction of the
Mn and
Mw of PLA [
36]. Hydrolytic degradation is a well-known degradation mechanism of PLA due to the presence of moisture. The hydrolysis of PLA begins by the diffusion of water molecules into the amorphous portion of PLA, resulting in the cleavage of the ester bonds [
37]. Thermal degradation of PLA during processing is also related to the hydrolysis of residual water, main-chain scission, and intra and intermolecular transesterification. A reduction of
Mn for PLA by hydrolysis was reported in the presence of water, even at low temperatures [
38]. The presence of low molecular weight additives, such as glycerol, has a negative impact on the molecular weight of PLA due to thermal degradation or the hydrolysis of polyester chains.
The production of films for characterization required a two-step processing method. In this case, the use of different temperatures and shear and mixing conditions allow for a reduction of molecular weight. Taubner and Shishoo reported a reduction of
Mn for PLA during melt extrusion using a double-screw extruder influenced by the processing temperature and residence time [
39].
In the case of PLA-GRH, the values of Mn and Mw were similar to those of PLA-c, showing that the addition of GRH nanoplatelets did not affect the PLA’s structure. In the case of the final reactive blend, PLA-g-TPCS-GRH, this resulted in a ~30% decrease of Mn and Mw with respect to PLA due to the dual processing steps of the composite blends: first, to produce the reactive blend and then due to the addition of the GRH nanoplatelets and reprocessing. This reduction is associated with thermal conditions inherent with the processing in the twin-screw and single extruders. Nonetheless, the values of PI showed a narrow distribution of the molecular weight for all the samples: PI ≤ 2.
3.2. Morphology of the Films
SEM images of the PLA blends were obtained to understand the grade of compatibilization between PLA and TPCS and the incorporation of GRH nanoplatelets. An optimal distribution of GRH nanoplatelets should play an important role in enhancing the barrier and mechanical properties of nanocomposite blends. The morphology of the fracture surface for the evaluated blends is shown in
Figure 1.
Figure 1a shows the surface of PLA-c.
Figure 1b shows the reactive blend of PLA-
g-TPCS after removing the TPCS phase; good compatibilization between PLA and TPCS was achieved in the reactive blend by the incorporation of MA and L101. Similar results for these reactive blends were reported by Detyothin et al. [
17]. The proportion of cavities observed due to the removal of TPCS is in accordance with the proportion used for the production of the reactive blend. The compatibilization achieved allowed for good homogenization during the processing step using a twin-screw extruder. The incorporation of GRH nanoplatelets into neat PLA is presented in
Figure 1c, where a small distribution of GRH nanoplatelets in the PLA matrix is in accordance with the low load of GRH used in the production (0.1 wt %).
Figure 1d shows the presence and distribution of GRH nanoplatelets in the matrix of the compatibilized blend obtained with PLA and TPCS. By comparing
Figure 1c,d, it appears that the GRH nanoplatelets are mostly located as flakes in the interface of PLA and TPCS.
Figure S1 (
supporting information available online) shows the graphene nanoplatelets powder used as a reinforcement and the formation of agglomerates. The TPCS domain was removed in
Figure 1d, and it is apparent that the GRH nanoplatelets are embedded in the cavities surrounded by the PLA matrix. This observation may help explain the mechanical property results whereby the GRH nanoplatelets increased the tensile strength of PLA-GRH with respect to neat PLA but also increased the elongation at break of the reactive blend produced with TPCS.
Figure 1e,f reveal further details of how the GRH nanoplatelets are inserted in both the PLA matrix and the PLA-
g-TPCS-GRH. An uneven distribution of nanoplatelets (but good compatibilization and adhesion) in the matrix is evident.
Figure 1e,f show the clustering or agglomeration of several GRH nanoplatelets, which could affect the barrier and mechanical properties. The presence of flakes of GRH in the matrix of PLA-c and PLA-
g-TPCS exhibiting a surface structure with cracks was also reported by Gao et al. [
40] working with nanocomposites of PLA and GRH nanoplatelets at concentrations between 5 and 15 wt %. Pinto et al. also reported deficient exfoliation of GRH nanoplatelets with aggregations of 5 to 10 sheets, as observed by SEM, and overlapping of nanoplatelets as observed by optical microscopy [
26]. How the distribution of GRH nanoplatelets in the polymer matrix affected the mechanical properties of the films is described in the next section.
Table 4 and
Figure 2 present the surface roughness of the PLA films as evaluated by AFM. The PLA-
g-TPCS and PLA-
g-TPCS-GRH films had higher values of surface roughness in comparison with the PLA-c and PLA-GRH films. This greater roughness could be attributed to the presence of the starch matrix in both reactive blends.
Figure 2 shows a smooth surface for PLA-c and PLA-GRH. Roughness values obtained by profilometry are larger than those obtained by AFM due to the larger scan area; however, the values follow the same trend (i.e., roughness for PLA-c and PLA-GRH are lower than for PLA-
g-TPCS and PLA-
g-TPCS-GRH.).
Figure 1a,b allow for a comparison of the difference observed in roughness by AFM due to the presence of the TPCS phase. An increase of roughness could be attributed to irregular shapes of starch granules as can be observed for the cavities where these granules were immersed. The creation of a topography with different values of roughness could be due to the presence of two different phases that were compatibilized.
The presence of GRH nanoplatelets could not be significantly affecting the roughness of the PLA films due to the low load used for film production, and its effect is masked in the reactive blends due to the effect of the TPCS phase. Pinto et al. evaluated the topography of PLA with GRH nanoplatelets as a nano-based material and reported an increase in roughness with a higher concentration of GRH nanoplatelets (0.4 wt %) [
41].
3.3. Tensile Properties
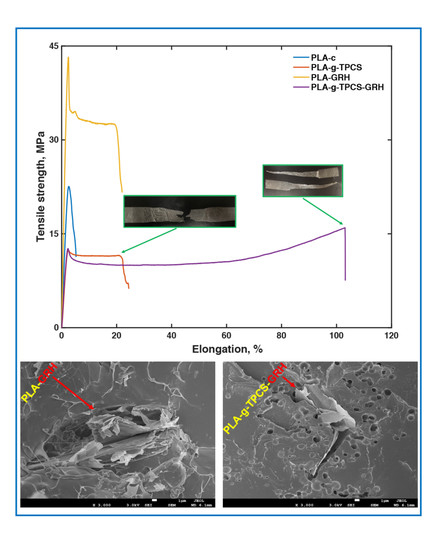
An analysis of the tensile testing of the PLA films, evaluated in the machine direction, reveals a characteristic brittle behavior for PLA-c, with tensile strength values of ~25 MPa and elongation at break values around 9% (
Table 5 and
Figure 3). However, the introduction of GRH nanoplatelets to the PLA matrix resulted in improvements in both tensile strength and elongation at break of ~75% and 130%, respectively. Others have reported similar values of tensile strength when GRH nanoplatelets were used as a nanofiller for PLA [
42]. Chieng et al. [
43] reported tensile strength values of ~60 MPa for PLA with 0.3 wt % of GRH. Valapa et al. [
27], using expandable graphite as reinforcement, found similar increments for elongation at break of PLA, but the increment for tensile strength was small with the same load (0.1 wt %).
The Young’s modulus increased by ~100% with respect to neat PLA, indicating the stiffness behavior of the incorporated nanofiller. Reactive blending using MA and L101 to obtain better compatibilization between PLA and TPCS resulted in a blend with good elongation at break (~25%) but a ~50% reduction in tensile strength with respect to PLA-c. Similar values have been previously reported for these blends [
17]. The addition of GRH nanoplatelets to the reactive blend of PLA-
g-TPCS resulted in an important improvement in the elongation at break (values larger than 100%), showing an increment of ~300% with respect to PLA-
g-TPCS. Values of toughness showed a significant improvement for PLA-
g-TPCS-GRH, around ~900% with respect to PLA-c.
Figure 3 insets are images of the films (PLA-
g-TPCS and PLA-
g-TPCS-GRH) after the tensile testing. The nanofiller incorporated did not improve the tensile strength of the reactive blend.
Figure 3 indicates that at least two mechanisms were acting when PLA-
g-TPCS-GRH films were tested: a well-identified yield point and a strain hardening behavior. In
Figure 1, the presence of gaps between the GRH nanoplatelets and the polymer matrix in the fractured surface of PLA-
g-TPCS-GRH could be identified. Furthermore,
Figure S2 shows the SEM images of the films after the tensile test. The cavities generated in the PLA-
g-TPCS polymer matrix during the tensile test can be observed, which could be one of the main reasons for the improvement in the elongation at break (
Figure S2b,e). Due to the incorporation of GRH nanoplatelets, when the material is under tension, the fractures created in the surface of the polymer matrix could find a free propagation path or a flake of GRH nanoplatelets. Since the GRH nanoplatelets are stiff materials, in the second case the fracture is forced to find an alternative path that continues with the propagation of the fracture and breaks the material during plastic deformation. Thus, an increase of the deformation energy and toughening is observed due to the addition of GRH, which is finally translated into high values of elongation at break [
27,
44].
The presence of GRH nanoplatelets, even at a low concentration, is enough to create a crack-bridging mechanism during tension [
45]. This mechanism could increase the fracture toughness of a nanocomposite, and its efficiency is important when the nanofiller has a high value of aspect ratio [
44]. One of the most significant properties of GRH nanoplatelets is their high aspect ratio. Since the adhesion of the GRH nanoplatelets to the PLA matrix is strong—as shown in the SEM images,
Figure 1d and
Figure S2f,l—and the nanoplatelets can act as a bridge between two fracture surfaces, they are avoiding or delaying the pullout and thus increasing the fracture energy, as was also demonstrated for other fillers [
44,
45,
46].
On the other hand, it has been reported that when PLA was loaded with GRH nanoplatelets above 0.3 wt %, the elongation at break decreased and that was attributed to the large load of GRH nanoplatelets restricting the mobility of the polymer chains [
42,
47]. A high load of GRH nanoplatelets in a polymer matrix allows for the restack of nanosheets due to a Van der Waals force. As a consequence, under tension the primary effect will be the slippage of the graphene nanosheets with lower values of tensile strength [
47].
Gao et al. [
40] also concluded that the incorporation of graphene nanoplatelets (~15 nm) at a higher load (e.g., 5 to 10%) reduced the mobility of the PLA chains in the composite and increased its brittle behavior, which could be attributed to some aggregation of nanoplatelets due to the high concentration.
3.4. Thermal Properties
Figure 4 shows the TGA results for the evaluated samples. No significant difference in onset decomposition temperature was observed among neat PLA, PLA-
g-TPCS, and samples with the GRH nanoplatelets incorporated into these matrixes; all samples had an onset temperature between 310–320 °C. An early change (before the onset temperature) was depicted, mainly due to the presence of the TPCS phase and the presence of glycerol. The incorporation of a nanofiller has been shown to enhance composite thermal stability; this was evident for PLA-GRH, but this was not evident for the addition of GRH nanoplatelets at low load (i.e., 0.1 wt %) in the PLA-
g-TPCS blends. Chieng et al. [
42], working with a plasticized PLA reinforced with GRH nanoplatelets, reported a similar value for the onset decomposition temperature. The addition of a load of GRH nanofiller above 0.5 wt % into the PLA matrix was shown to significantly improve thermal stability [
42]. The incorporation of GRH nanoplatelets at higher loads could work as a heat barrier, shifting the decomposition of the composite to higher temperatures, and also, due to its high aspect ratio, create a barrier for volatile degradation products present in the nanocomposite [
48]. Similar values of onset decomposition temperature (
Tonset) and maximum decomposition temperature (
Tdmax) were observed for PLA and the reactive blends with and without the addition of GRH nanoplatelets (
Table 6). Residual values observed are due to the decomposition of organic matter of TPCS and the formation of ash [
49]. A lower residual was observed for PLA-GRH. This could be associated with the fact that GRH nanoplatelets are thermally conductive, and thus could improve the loss of residual mass of this blend at high temperature. Additionally, there is no presence of ash derived from the cassava starch.
The DSC results for the second heating cycle are presented in
Table 6 and
Figure 5. With respect to PLA-c, reductions in
Tg of ~10 °C for the reactive blend PLA-
g-TPCS and ~5 °C for the reactive blend with GRH nanoplatelets were observed. However, PLA-GRH had a similar
Tg to PLA-c. Valapa et al. [
27] also reported that a low content of expandable graphite in PLA composites did not affect the
Tg, concluding that an incorporation of a low amount of reinforcement does not affect the mobility and reduction of PLA chains. On the other hand, lower
Tg values have been reported for plasticized PLA nanoreinforced with GRH nanoplatelets [
42], although the loads were higher (0.3 to 1.0 wt %). A similar reduction in
Tg has been reported due to the presence of a TPCS phase and the use of MA and L101 [
17]. The incorporation of GRH nanoplatelets in the PLA-
g-TPCS increased its
Tg compared with the unreinforced blends; this finding could be correlated with the tensile test results, whereby the GRH nanoplatelets acted as a reinforcement of the blends, reducing PLA chain mobility. The reactive blends had lower
Tm values in comparison to PLA-c, and this decline could be associated with the reduction in
Mn, which has a larger effect on
Tm than on
Tg. PLA-GRH exhibited a small decrease in
Tm.
The values of crystallization, as determined by the second heating cycle, remained stable for all of the blends, which are mostly amorphous. The presence of GRH nanoplatelets did not modify the crystallization behavior of PLA, as observed in
Figure S3. A small crystallization peak is shown at 16.4° and assigned to the plane (200)/(110) of the α-crystal of PLA, confirming the presence of an ordered region due to the presence of the nanofiller [
32]. However, the blending with TPCS disrupts the crystallization process, creating two types of crystal forms (α and α’) as previously reported [
50], which are difficult to crystallize. The XRD patterns (
Figure S3) exhibited a fully amorphous behavior for all of the films produced, with a crystallization peak for PLA-GRH at 16.4° and a peak showing the presence of GRH nanoplatelets in PLA-GRH and PLA-
g-TPCS-GRH at 26.5° (corresponding with the
d002 spacing of graphite). The presence of this peak at 26.5° confirms that a high percentage of GRH nanoplatelets were intercalated in the polymer matrix. The thermal parameters obtained from the first heating cycle of the DSC (
Table S1) showed that the crystallinity of all of the samples was mostly amorphous.
Figure 6a shows that the storage moduli,
Eʹ, is reduced in the range of 35 to 60 °C due to the addition of TPCS phase concerning the PLA-c, indicating a reduction of the elastic region for PLA-
g-TPCS. The reduction observed for
E’ upon the incorporation of GRH in PLA is due to the increase in toughening and the more plastic behavior of PLA-GRH. The
E’ represents the stiffness of the viscoelasticity of PLA and is proportional to the energy stored during the loading cycle. Since the addition of GRH improves the Young’s Modulus of PLA-GRH samples and also the toughness of the samples through the crack-bridging mechanism as previously described, the increase of the Young’s Modulus is due to the addition of the GRH nanoplatelets (the modulus is around 1 TPa for GRH), and the simultaneous reduction of
E’ is an indication of a less elastic and more plastic deformation with the temperature of the PLA-GRH sample. This incorporation of GRH into PLA-
g-TPCS has less effect on the reduction of
E’ due to the presence of the plastic TPCS phase. With the increment of temperature after 60 °C, a slow decrease is observed for
Eʹ until it reaches the glass transition region where the drop of
E’ is important. Above the glass transition region, the values for
Eʹ are similar for all of the evaluated samples. An important reduction of
Eʺ is observed for the reactive blends with respect to PLA. Thus, the reactive blends showed a tough behavior with less energy dissipated during deformation.
The
Tg values have been reported to be higher when estimated by DMA than by DSC [
51]. Nevertheless, the
Tg trend observed in the DMA was the same as that observed in the DSC analysis, with similar values for PLA-c and PLA-GRH and a shift to lower temperatures for PLA-
g-TPCS and PLA-
g-TPCS-GRH. This shift is associated with the enhanced chain mobility of PLA due to the TPCS phase and the plasticizing effect achieved by reactive compatibilization. The plasticization effect could be described as the softening of the blend due to the presence of the glycerol. However, the decrease of the tan delta peak with the addition of GRH indicates that the polymer chains during the transition region are less restricted by the GRH nanoplatelets. Others have suggested that the reinforcement due to the addition of GRH nanoplatelets leads to a restriction of the chain mobility of the polymer [
52]. A similar reduction of tan delta peaks was reported by Jonoobi et al. [
53] when using cellulose nanofibers to reinforce PLA.
3.5. Optical Properties of Films
Transmittance of the characterized films is presented in
Figure 7. The films can be divided into two groups. In the first group, comprising PLA-c and PLA-GRH, a ~10% reduction in transmittance between 250 and 880 nm was observed between the films due to the presence of GRH nanoplatelets. The films in the second group, PLA-
g-TPCS and PLA-
g-TPCS-GRH, had similar transmittance values, but these values were ~75% lower with respect to the first group. This phenomenon is mainly due to the presence of TPCS in the matrix of PLA-
g-TPCS and PLA-
g-TPCS-GRH, as can be observed in
Figure S4, and which was discussed previously in terms of the increase of roughness in these films, as determined by AFM. A full characterization of the color and opacity of the film samples is provided in
Table S2. The incorporation of 0.1% of nanofiller into PLA has shown similar characteristics as those reflected in this work [
27].
It is also important to note that the presence of GRH in PLA-GRH and PLA-
g-TPCS-GRH plays an important role in the electrical properties of these materials. The values of electrical resistivity for the films are provided in
Table S3, and indicate that sample conductivity increases with the addition of very low amounts of GRH nanoplatelets. Higher values of conductivity were reported by Gao et al. [
40] working with higher loads of GRH nanoplatelets (5–15%).