1. Introduction
Contamination by microorganisms such as bacteria, fungi, and algae is a key issue in medicine [
1], pharmaceutical production [
2], water purification systems [
3], food packaging [
4], and various other fields [
5,
6]. Artificial materials lack defense against microbial growth, allowing microbes to attach to the surface and form a biofilm [
7]. One way to prevent the biofilm formation is the usage of disinfectants to keep the surface sterile. Common disinfectants include low molecular weight substances such as alcohols, aldehydes, quaternary ammonium compounds, silver compounds, peroxygens, and bisphenols [
8]. However, these classes of antimicrobial substances need to be applied regularly, leading to the development of resistance in the microbial strains [
9]. Another way to prevent microbial growth is the coating of surfaces with antimicrobial substances that either kill microorganisms on contact or repel the attachment of microbes [
10]. Nevertheless, the leaching of biocides from those coatings causes the same previously mentioned problems. An attractive alternative to low molecular weight biocides are antimicrobial polymers, because they are non-volatile, chemically stable, and can be used as non-releasing additives [
11]. These polymers are prepared either by (co-)polymerization of functionalized monomers or by post-polymerization functionalization [
12]. Though, the antimicrobial properties are derived from a variety of functionalities such as biguanides [
13,
14], benzoate esters and benzaldehydes [
15], or poly(acrylic acid) [
16], the most common active moieties are based on a combination of quaternary ammonium, pyridinium, or phosphonium groups and hydrophobic functionalities [
17,
18,
19]. The proposed and commonly accepted mechanism for these types of polymers features the electrostatic interaction between the cationic moieties and the negatively charged bacterial cell membrane and the disruption of the cell membrane by the hydrophobic groups, leading to leakage and, subsequently, cell death [
20,
21]. However, due to the heterogeneity and bacterial strain specificity of the cell wall composition, the efficacy of antimicrobial polymers is species-dependent. The outer part of the cell wall of Gram-positive bacteria is composed of about 90% peptidoglycan. In Gram-negative bacteria, the peptidoglycan layer accounts for approximately 20% of the cell envelope, being located between the outer membrane and inner cell membrane. Before reaching the inner cell membrane, polymers interact with the negatively charged components of the outer part of the bacterial cell envelope, e.g., teichoic acids in the thick peptidoglycan layers of Gram-positive bacteria, and phospholipids and lipopolysaccharides in the outer membrane of Gram-negative bacteria [
22,
23]. The effectiveness of the antimicrobial cationic–hydrophobic polymers is dependent on various factors [
24]. One factor is the molecular weight of the polymer. Ikeda et al. synthesized polymethacrylates with pendant biguanide units and various molecular weights and tested the antimicrobial activity against
Staphylococcus aureus, reaching an optimal activity at molecular weights between 50 and 100 kDa [
25]. Kanazawa et al. showed an increase in biocidal activity of poly[tributyl(4-vinylbenzyl)phosphonium chloride] against
S. aureus with increasing molecular weight [
26]. Based on the described mechanism, they assume that a higher molecular weight and a consequential higher charge density enhance the adsorption of the polymers to the cell membrane. A stronger adsorption leads to a stronger disruption of the cell membrane and, thus, to a higher activity of the polymer. However, Lienkamp et al. found that when the molecular weight reaches a threshold value, the efficacy of the polymers decreases [
27]. Additionally, Locock et al. reported the reverse effect on guanylated polymethacrylates [
28]. The presented polymers showed higher antimicrobial activity at lower molecular weights, proving that the efficacy of antimicrobial polymers does not exclusively depend on their molecular weight. A second factor for the antimicrobial behavior is the alkyl chain length of the hydrophobic moiety. However, the optimal alkyl chain length is different for different types of polymers. Pasquier et al. prepared branched poly(ethylene imine)s with pendant ammonium and alkyl functionalities, ranging from C
6 to C
16. An increase in the alkyl chain length lead to an increase in the antimicrobial activity against
E. coli [
29]. The activity was further enhanced by He et al., attaching the hydrophobic chain directly to the ammonium group [
30]. The opposite influence of aliphatic side chains was reported by Xu et al. on comb-like ionenes [
31]. Here, a decrease in the alkyl chain length lead to an increase in the antimicrobial activity against
E. coli. On the other hand, Chen et al. prepared poly(propylene imine) dendrimers with alkyl chains ranging from C
8 to C
16, showing a parabolic dependence between the biocidal effect and the alkyl chain length, with the highest activity at C
10 [
32].
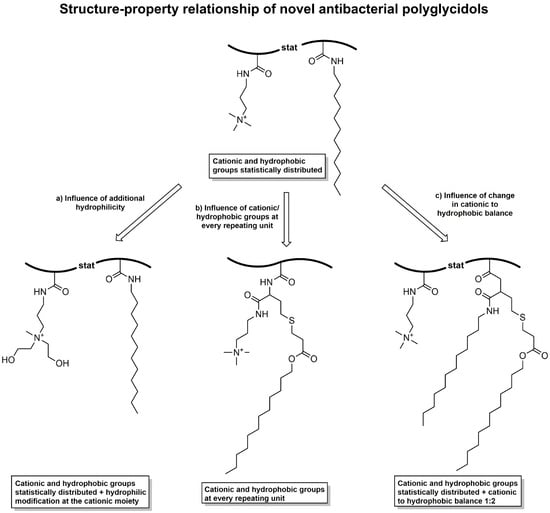
In the current work, we present the preparation of various antimicrobial polymers based on polyglycidol with quaternary trimethylammonium groups as the cationic moiety and dodecyl chains as the hydrophobic part. Polyglycidol is a highly functional polymer with a hydroxy group in every repeating unit, allowing various further modifications [
33,
34]. It is non-toxic, soluble in aqueous media, and licensed by the Food and Drug Administration (FDA) [
35,
36]. The cationic and hydrophobic side chain functionalities were distributed statistically along the polymer backbone (cationic–hydrophobic ratio of 1:1). This functionalized polyglycidol was compared to (i) a polyglycidol modified with hydrophilic hydroxyethyl functionalities at the cationic center; (ii) a polyglycidol with cationic and hydrophobic moieties at every repeating unit; and (iii) a polyglycidol with a cationic–hydrophobic balance of 1:2. All polymers were tested in regard to their antimicrobial properties against
Escherichia coli and
Staphylococcus aureus to examine a possible relationship between the structure and the biocidal effect of the polymer.
2. Materials and Methods
2.1. Materials
Potassium tert-butoxide (1 M solution in THF, Sigma Aldrich Chemie GmbH, Steinheim, Germany), diglyme (≥99%, extra dry, over molecular sieves, Thermo Fisher Acros Organics, Geel, Belgium), pyridine (99.5%, extra dry, over molecular sieves, Thermo Fisher Acros Organics, Geel, Belgium), phenyl chloroformate (>97%, Sigma Aldrich Chemie GmbH, Steinheim, Germany), 4-nitrophenyl chloroformate (>98%, TCI Deutschland GmbH, Eschborn, Germany), 3-(dimethylamino)-1-propylamine (99%, Thermo Fisher Acros Organics, Geel, Belgium), N-(3-aminopropyl)diethanolamine (>90%, TCI Deutschland GmbH, Eschborn, Germany), dodecylamine (98%, Sigma Aldrich Chemie GmbH, Steinheim, Germany), 4-dimethylaminopyridine (>98%, Sigma Aldrich Chemie GmbH, Steinheim, Germany), dl-homocysteine thiolactone hydrochloride (98%, abcr GmbH, Karlsruhe, Germany), triethylamine (≥99.5%, anhydrous, Sigma Aldrich Chemie GmbH, Steinheim, Germany), dodecyl acrylate (>98%, TCI Deutschland GmbH, Eschborn, Germany), methyl iodide (>99%, Sigma Aldrich Chemie GmbH, Steinheim, Germany), tetrahydrofuran (99.8%, stabilizer free, extra dry, Thermo Fisher Acros Organics, Geel, Belgium), chloroform (99.9%, extra dry, over molecular sieves, Thermo Fisher Acros Organics, Geel, Belgium), N,N-dimethylformamide (99.8%, extra dry, over molecular sieves, Thermo Fisher Acros Organics, Geel, Belgium), methanol (≥99.8%, p.a., Th. Geyer CHEMSOLUTE®, Renningen, Germany), and dichloromethane (≥99.8%, anhydrous, Sigma Aldrich Chemie GmbH, Steinheim, Germany) were used as received.
The 3-phenyl-1-propanol (99%, Sigma Aldrich Chemie GmbH, Steinheim, Germany) was stirred with calcium hydride for 24 h and then distilled. Ethoxyethyl glycidyl ether (EEGE) was synthesized from 2,3-epoxypropan-1-ol (glycidol, 96%, Sigma Aldrich Chemie GmbH, Steinheim, Germany) and ethyl vinyl ether (99%, Sigma Aldrich Chemie GmbH, Steinheim, Germany) according to Fitton et al. [
37], purified by distillation, and stored under a nitrogen atmosphere over a molecular sieve (3 Å). Polyglycidol (PG) (
1) was synthesized according to literature [
38].
Water-sensitive reactions were carried out in a nitrogen atmosphere. Nitrogen (Linde 5.0) was passed over a molecular sieve (4 Å) and finely distributed potassium on aluminum oxide.
2.2. Bacteria
To determine the antibacterial activity, polymers were tested against the Gram-negative bacterium Escherichia coli (DSM498) and the Gram-positive bacterium Staphylococcus aureus (ATCC6538). Overnight cultures of E. coli and S. aureus with defined bacterial count in Mueller–Hinton broth (Sigma Aldrich Chemie GmbH, Steinheim, Germany, pH = 7.4 ± 0.2) were used as inoculate in the antibacterial test.
2.3. Antibacterial Tests of Polymer Solutions
The antibacterial activity of the polymers in solution was determined by measuring the minimal inhibitory concentration (MIC) using the test bacteria mentioned above. Suspensions of strains with known colony forming units (CFU; 1 × 10
6 ‒2 × 10
6 CFU·mL
−1) were incubated at 37 °C in nutrient solutions (Mueller–Hinton Broth, MHB) with different concentrations of the polymer samples. The polymer samples were solubilized in bidistilled water and added to the nutrient solution at a constant ratio of 1:10. The growth of the bacteria was followed during the incubation over 20 h by measuring the optical density at 612 nm every 30 min (with 1400 s of shaking at 100 rpm per 30 min cycle by using a microplate reader/incubator (TECAN Infinite 200 Pro, Tecan Trading AG, Männedorf, Switzerland). The testing is performed with defined concentrations specifically for each polymer until, within the monitoring time of 20 h, no bacterial growth curve is recorded. All experiments were performed in triplicate duplicates and MIC determination was repeated on three different days. The polymers were not sterilized. Sterile controls (defined polymer concentrations in nutrient solution without bacteria) were assessed in every growth curve monitoring testing series. MICs were determined according to broth microdilution in 96-well microtitre plates [
39]. Antimicrobial polymer testing reference polymers/controls ε-polylysine and polyhexanide (poly(hexamethylene biguanide)) were used.
The minimal inhibitory concentration (MIC) corresponds to the concentration of the test substance at which a complete inhibition of the growth of the inoculated bacteria was observed by comparison with control samples without test substance.
2.4. Hemolytic Activity
Hemolytic activity was assessed according to literature [
40]. Human erythrocytes (from healthy donors, red blood cells (RBC), 0, Rh positive, citrate-stabilized) were obtained by centrifugation (3500 rpm, 12 min) to remove plasma, washed three times in PBS (0.01 M phosphate buffered saline, Sigma Aldrich Chemie GmbH, Steinheim, Germany), and diluted in PBS to obtain a stock solution of 2.5 × 10
8–3.0 × 10
8/mL RBC. Solutions of defined polymer concentration (250 µL) were pipetted into 250 μL of the stock solution; the final amount of RBC being 1.2 × 10
8 –1.5 × 10
8 RBC/mL. The RBC were exposed for 60 min at 37 °C under 3D-shaking; centrifuged thereafter (4000 rpm, 12 min) and the absorption of the supernatant (diluted 10-fold in PBS) was determined at 414 nm in a microplate reader. As reference solutions, (i) PBS for determining spontaneous hemolysis and (ii) 1% Triton X-100 for 100% hemolysis (positive control) were used. Hemolysis was plotted as a function of polymer concentration and the hemolytic activity was defined as the polymer concentration that causes 50% hemolysis of human RBC relative to the positive control (HC
50).
2.5. Measurements
1H NMR and 13C NMR were recorded on a Bruker DPX-400 FT-NMR spectrometer (Bruker Corporation, Billerica, MA, USA) at 400 and 101 MHz, respectively. Deuterated chloroform (CDCl3), deuterated dimethyl sulfoxide (DMSO-d6), deuterated acetone (acetone-d6), and deuterated methanol (MeOD) were used as solvents. The residual solvent signal was used as internal standard. Coupling constants Jxy are given in Hz.
Molecular weights (Mn,SEC) and molecular weight distributions (Đ) were determined by size exclusion chromatography (SEC). SEC analyses were carried out with DMF as eluent. SEC with DMF (HPLC grade, VWR) as eluent was performed using an Agilent 1100 system (Agilent Technologies, Santa Clara, CA, USA) equipped with a dual RI-/Visco detector (ETA-2020, WGE Dr. Bures GmbH & Co KG, Dallgow-Doeberitz, Germany). The eluent contained 1 g·L−1 LiBr (≥99%, Sigma Aldrich Chemie GmbH, Steinheim, Germany). The sample solvent contained traces of distilled water as internal standard. For cationic samples, the eluent contained 2 g·L−1 LiBr and 2 g·L−1 tris(hydroxymethyl)aminomethane (TRIS, ultrapure grade, ≥99.9%, Sigma Aldrich Chemie GmbH, Steinheim, Germany). One pre-column (8 mm × 50 mm) and four GRAM gel columns (8 mm × 300 mm, Polymer Standards Service, Mainz, Germany) were applied at a flow rate of 1.0 mL·min−1 at 40 °C. The diameter of the gel particles measured 10 µm, the nominal pore widths were 30, 100, 1000, and 3000 Å. Calibration was achieved using narrowly distributed poly(methyl methacrylate) standards (Polymer Standards Service, Mainz, Germany). Results were evaluated using the PSS WinGPC UniChrom software (Version 8.1.1, PSS Polymer Standards Service GmbH, Mainz, Germany).
Dialysis was performed in methanol using Biotech CE Tubing (MWCO: 100–500 D, 3.1 mL·cm−1, Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA) and Biotech RC Tubing (MWCO: 1 kD, 6.4 mL·cm−1, Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA), respectively. The membrane was washed for 15 min in water before use to remove the sodium azide solution.
2.6. Synthesis of Poly(glycidyl phenyl carbonate) (P(GPC)27) (2)
PG27 (1) (2.018 g, 27.24 mmol OH) was dissolved in pyridine (18.87 mL), and a solution of phenyl chloroformate (4.692 g, 29.97 mmol) in dichloromethane (17.5 mL) was added in 30 min at 0 °C using a syringe pump. The reaction mixture was allowed to warm to room temperature and stirred for 20 h. The precipitate was removed by filtration. The solution was washed with water (15 mL), 1 M HCl solution (aq.) (3 × 15 mL), and saturated NaCl solution (aq.) (15 mL). The organic phase was dried over Na2SO4, filtrated, and the solvent removed under reduced pressure. Polymer 2 was obtained as a brown viscous liquid (3.650 g, 69%). Mn,NMR = 5243 g·mol−1, Mn,SEC = 4800 g·mol−1, Ð = 1.14. 1H NMR (400 MHz, CDCl3) (2): δ = 1.79 (m, ArCH2CH2), 2.57 (t, 3JHH = 7.8 Hz, ArCH2CH2), 3.45–3.87 (m, ArCH2CH2CH2, OCH2CH(CH2OC=OOPh)O), 4.08–4.42 (m, OCH2CH(CH2OC=OOPh)O), 6.98–7.29 (m, ArCH2CH2, (OC=OOPh)O) ppm. 13C NMR (101 MHz, CDCl3) (2): δ = 31.2 (ArCH2CH2), 32.3 (ArCH2CH2), 67.7–69.4 (ArCH2CH2CH2, OCH2CH(CH2OC=OOPh)O), 77.4 (OCH2CH(CH2OC=OOPh)O), 121.0 (OC=OOPh)O), 126.1 (ArCH2, OC=OOPh)O), 128.4 (ArCH2), 128.5 (ArCH2), 129.6 (OC=OOPh)O), 141.8 (ArCH2), 151.1 (OC=OOPh)O), 153.6 (OC=OOPh)O) ppm.
2.7. Synthesis of Poly(glycidyl 3-dimethylaminopropylcarbamate-co-glycidyl dodecylcarbamate) (P(GDMAPA15-co-GDDA12)) (3)
P(GPC)27 (2) (1.509 g, 7.77 mmol carbonate) was dissolved in tetrahydrofuran (15 mL) and a solution of 3-(dimethylamino)-1-propylamine (0.397 g, 3.89 mmol) and dodecylamine (0.721 g, 3.89 mmol) in tetrahydrofuran (15 mL) was added in 1 h at 0 °C using a syringe pump. The reaction was allowed to warm to room temperature and stirred for 42 h. The solvent was removed under reduced pressure and the polymer purified by dialysis in methanol. Polymer 3 was obtained as a yellowish viscous liquid (1.153 g, 62%). Mn,NMR = 6459 g·mol−1, Mn,SEC = 7600 g·mol−1, Ð = 1.38. 1H NMR (400 MHz, CDCl3) (3): δ = 0.84 (t, 3JHH = 7.0 Hz, NHCH2CH2(CH2)9CH3), 1.21 (s, NHCH2CH2(CH2)9CH3), 1.34–1.51 (m, NHCH2CH2(CH2)9CH3), 1.54–1.68 (m, NHCH2CH2CH2N(CH3)2), 1.77–1.89 (m, ArCH2CH2), 2.16 (s, NHCH2CH2CH2N(CH3)2), 2.27 (t, 3JHH = 6.7 Hz, NHCH2CH2CH2N(CH3)2), 2.63 (t, 3JHH = 7.6 Hz, ArCH2CH2), 3.01–3.11 (m, NHCH2CH2(CH2)9CH3), 3.12–3.21 (m, 2H, NHCH2CH2CH2N(CH3)2), 3.45–3.79 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.86–4.36 (m, OCH2CH(CH2OC=ONHR)O), 5.88 (br. s, NH), 6.17 (br. s, NH), 7.09–7.25 (m, ArCH2CH2) ppm. 13C NMR (101 MHz, CDCl3) (3): δ = 14.2 (NHCH2CH2(CH2)9CH3), 22.7 (NHCH2(CH2)10CH3), 27.0 (NHCH2CH2CH2N(CH3)2, 27.5–32.0 (NHCH2(CH2)10CH3, ArCH2CH2), 39.9 (NHCH2(CH2)10CH3), 41.2 (NHCH2CH2CH2N(CH3)2), 45.5 (NHCH2CH2CH2N(CH3)2), 57.6 (NHCH2CH2CH2N(CH3)2), 64.2–70.0 (ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 78.0 (OCH2CH(CH2OC=ONHR)O), 125.8 (ArCH2), 128.4 (ArCH2), 128.5 (ArCH2), 141.9 (ArCH2), 156.7 (OCH2CH(CH2OC=ONHR)O) ppm.
2.8. Synthesis of Poly(glycidyl 3-aminopropyldiethanolcarbamate-co-glycidyl dodecylcarbamate) (P(GAPDEA16-co-GDDA11)) (4)
P(GPC)27 (2) (0.707 g, 3.51 mmol carbonate) was dissolved in tetrahydrofuran (10 mL) and a solution of N-(3-aminopropyl)diethanolamine (0.284 g, 1.75 mmol), and dodecylamine (0.325 g, 1.75 mmol) in tetrahydrofuran (5 mL) was added in 1 h at 0 °C using a syringe pump. The reaction was allowed to warm to room temperature and stirred for 42 h. The solvent was removed under reduced pressure and the polymer purified by dialysis in methanol. Polymer 4 was obtained as a colorless viscous liquid (0.655 g, 66%). Mn,NMR = 7337 g·mol−1, Mn,SEC = 14,100 g·mol−1, Ð = 3.56. 1H NMR (400 MHz, MeOD) (4): δ = 0.92 (t, 3JHH = 7.0 Hz, NHCH2CH2(CH2)9CH3), 1.31 (s, NHCH2CH2(CH2)9CH3), 1.44–1.59 (m, NHCH2CH2(CH2)9CH3), 1.62–1.76 (m, NHCH2CH2CH2N(CH2CH2OH)2), 1.85–1.92 (m, ArCH2CH2), 2.55–2.74 (m, CH2N(CH2CH2OH)2), 3.06–3.14 (m, NHCH2CH2(CH2)9CH3), 3.15–3.23 (m, NHCH2CH2CH2N(CH2CH2OH)2), 3.62 (t, 3JHH = 5.4 Hz, CH2N(CH2CH2OH)2), 3.66–3.81 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 4.00–4.34 (m, OCH2CH(CH2OC=ONHR)O), 7.17–7.32 (m, ArCH2CH2) ppm. 13C NMR (101 MHz, MeOD) (4): δ = 14.6 (NHCH2CH2(CH2)9CH3), 23.8–30.9 (NHCH2CH2(CH2)9CH3), 28.2 (NHCH2CH2CH2N(CH2CH2OH)2), 33.1 (NHCH2CH2(CH2)9CH3), 40.1 (NHCH2CH2CH2N(CH2CH2OH)2), 42.0 (NHCH2CH2(CH2)9CH3), 53.6 (CH2N(CH2CH2OH)2), 57.6 (CH2N(CH2CH2OH)2), 60.8 (CH2N(CH2CH2OH)2), 65.3–79.3 (ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 129.4 (ArCH2), 129.6 (ArCH2), 158.7 (OCH2CH(CH2OC=ONHR)O) ppm.
2.9. Synthesis of Poly(glycidyl 3-trimethylaminopropylcarbamate-co-glycidyl dodecylcarbamate) (P(GTMAPA15-co-GDDA12)) (5)
P(GDMAPA15-co-GDDA12) (3) (0.147 g, 0.34 mmol –NMe2) was dissolved in THF (3.0 mL). Methyl iodide (0.058 g, 0.41 mmol) was added and the solution was stirred for 20 h at room temperature. Excess methyl iodide and the solvent were removed under reduced pressure and the polymer purified by dialysis in methanol. Polymer 5 was obtained as a yellowish crystalline solid (0.205 g, 89%). Mn,NMR = 10,111 g·mol−1, Mn,SEC = 4400 g·mol−1, Ð = 1.18. 1H NMR (400 MHz, DMSO-d6) (5): δ = 0.74–0.90 (m, NHCH2CH2(CH2)9CH3), 1.20 (s, NHCH2CH2(CH2)9CH3), 1.30–1.44 (m, NHCH2CH2(CH2)9CH3), 1.72–1.91 (m, NHCH2CH2CH2N+(CH3)3, ArCH2CH2), 2.55–2.62 (m, 3JHH = 7.6 Hz, ArCH2CH2), 2.85–2.99 (m, NHCH2CH2(CH2)9CH3), 3.00–3.17 (m, NHCH2CH2CH2N+(CH3)3, NHCH2CH2CH2N+(CH3)3), 3.26–3.43 (m, NHCH2CH2CH2N+(CH3)3), 3.44–3.74 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.82–4.19 (m, OCH2CH(CH2OC=ONHR)O), 7.10–7.30 (m, ArCH2CH2) ppm. 13C NMR (101 MHz, DMSO-d6) (5): δ = 13.8 (NHCH2CH2(CH2)9CH3), 22.0–28.9 (NHCH2(CH2)10CH3), 26.2 (NHCH2CH2CH2N+(CH3)3), 31.2 (NHCH2(CH2)10CH3), 37.3 (NHCH2CH2CH2N+(CH3)3), 52.2 (NHCH2CH2CH2N+(CH3)3), 63.2 (NHCH2CH2CH2N+(CH3)3), 68.6–77.0 (ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), OCH2CH(CH2OC=ONHR)O), 125.5 (ArCH2), 128.9 (ArCH2), 141.5 (ArCH2), 156.0 (OCH2CH(CH2OC=ONHR)O) ppm.
2.10. Synthesis of Poly(glycidyl 3-aminopropyldiethanolmethylcarbamate-co-glycidyl dodecylcarbamate) (P(GAPDEMA16-co-GDDA11)) (6)
P(GAPDEA16-co-GDDA11) (4) (0.216 g, 0.47 mmol –NEtOH2) was dissolved in THF (2.5 mL). Methyl iodide (0.081 g, 0.57 mmol) was added and the solution was stirred for 20 h at room temperature. Excess methyl iodide and the solvent were removed under reduced pressure and the polymer purified by dialysis in methanol. Polymer 6 was obtained as a slightly yellow solid (0.238 g, 84%). Mn,NMR = 9608 g·mol−1, Mn,SEC = not measurable. 1H NMR (400 MHz, MeOD) (6): δ = 0.96 (t, 3JHH = 6.3 Hz, NHCH2CH2(CH2)9CH3), 1.35 (s, NHCH2CH2(CH2)9CH3), 1.48–1.65 (m, NHCH2CH2(CH2)9CH3), 1.88–1.99 (m, ArCH2CH2), 2.05–2.23 (m, NHCH2CH2CH2N+(CH3(CH2CH2OH)2)), 2.70–2.78 (m, ArCH2CH2), 3.09–3.20 (m, NHCH2CH2(CH2)9CH3), 3.24–3.42 (m, NHCH2CH2CH2N+(CH3(CH2CH2OH)2)), 3.51–3.96 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O, CH2N+(CH3(CH2CH2OH)2)), 4.02–4.42 (m, OCH2CH(CH2OC=ONHR)O, CH2N+(CH3(CH2CH2OH)2)), 7.20–7.36 (m, ArCH2CH2) ppm. 13C NMR (101 MHz, MeOD) (6): δ = 14.5 (NHCH2CH2(CH2)9CH3), 23.7–31.0 (NHCH2CH2(CH2)9CH3), 28.0 (NHCH2CH2CH2N+(CH3(CH2CH2OH)2), 33.0 (NHCH2CH2(CH2)9CH3), 38.9 (NHCH2CH2CH2N+(CH3(CH2CH2OH)2)), 42.0 (NHCH2CH2(CH2)9CH3), 50.9 (NHCH2CH2CH2N+(CH3(CH2CH2OH)2), 56.8 (NHCH2CH2CH2N+(CH3(CH2CH2OH)2), 62.8–79.1 (ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 65.4 (CH2N+(CH3(CH2CH2OH)2), 129.4 (ArCH2), 129.6 (ArCH2), 158.7 (OCH2CH(CH2OC=ONHR)O) ppm.
2.11. Synthesis of Poly(glycidyl-4-nitrophenyl carbonate) (P(GNPC)27) (7)
PG27 (1) (2.012 g, 27.16 mmol OH) was dissolved in pyridine (18.85 mL). The 4-nitrophenyl chloroformate (6.023 g, 29.88 mmol) was dissolved in dichloromethane (20 mL) and added to the polymer solution via syringe pump in 30 min at 0 °C. The solution was stirred for 20 h at room temperature. The crude product was washed with water (30 mL), 1 M HCl (aq.) (3 × 30 mL), and saturated NaCl solution (aq.) (30 mL). The organic phase was separated, dried over Na2SO4 and precipitated in cold MeOH. The solvent was removed under reduced pressure and polymer 7 was obtained as a colorless solid (5.782 g, 89%). Mn,NMR = 6458 g·mol−1, Mn,SEC = 6600 g·mol−1, Ð = 1.41. 1H NMR (400 MHz, DMSO-d6) (7): δ = 1.69–1.83 (m, ArCH2CH2), 2.54–2.61 (m, ArCH2CH2), 3.54–3.95 (m, ArCH2CH2CH2, OCH2CH(CH2OC=OOArNO2)O), 4.15–4.56 (d, CH2OC=OOArNO2), 7.08–7.25 (m, ArCH2CH2), 7.42 (s, CH2OC=OOArNO2), 8.18 (s, CH2OC=OOArNO2) ppm. 13C NMR (101 MHz, DMSO-d6) (7): δ = 30.8 (ArCH2CH2), 31.6 (ArCH2), 68.2 (OCH2CH(CH2OC=OOArNO2)O), 76.4 (OCH2CH(CH2OC=OOArNO2)O), 76.5 (ArCH2CH2CH2), 76.6 (OCH2CH(CH2OC=OOArNO2)O), 122.3 (CH2OC=OOArNO2), 125.2 (CH2OC=OOArNO2), 125.7 (ArCH2), 128.2 (ArCH2), 128.3 (ArCH2), 141.6 (ArCH2), 145.0 (CH2OC=OOArNO2), 152.0 (OC=OO), 155.1 (CH2OC=OOArNO2) ppm.
2.12. Synthesis of Poly(glycidyl homocysteine thiolactonylcarbamate) (P(GHCTL)27) (8)
P(GNPC)27 (7) (1.020 g, 4.26 mmol carbonate) was dissolved in DMF (10 mL), and 4-(dimethylamino)pyridine (0.053 g, 0.43 mmol) and dl-homocysteine thiolactone hydrochloride (0.655 g, 4.26 mmol) were added. The mixture was cooled to 0 °C and triethylamine (0.862 g, 8.52 mmol) was added over 1 h via syringe pump. The solution was stirred for 20 h at room temperature. DMF was removed under reduced pressure at 50 °C and the crude product was precipitated in MeOH. Drying under reduced pressure at 50 °C gave polymer 8 as a slightly yellow solid (0.694 g, 75%). Mn,NMR = 5865 g·mol−1, Mn,SEC = 6900 g·mol−1, Ð = 1.44. 1H NMR (400 MHz, DMSO-d6) (8): δ = 1.74–1.83 (m, ArCH2CH2), 2.00–2.48 (m, C=OSCH2CH2CHR), 2.60 (t, 3JH,H = 7.5 Hz, ArCH2CH2), 3.17–3.45 (m, C=OSCH2CH2CHR), 3.47–3.75 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.86–4.23 (m, CH2OC=ONHR), 4.26–4.42 (m, C=OSCH2CH2CHR), 7.14–7.29 (Ar), 7.55 (br s, NH) ppm. 13C NMR (101 MHz, DMSO-d6) (8): δ = 26.5 (C=OSCH2CH2CHR), 29.8 (C=OSCH2CH2CHR), 30.9 (ArCH2CH2), 31.6 (ArCH2), 59.9 (CH2OC=ONHR), 63.7, 68.7, 77.2 (ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 79.2 (C=OSCH2CH2CHR), 128.3 (ArCH2), 128.4 (ArCH2), 156.0 (OC=ONHR), 205.7 (C=OSCH2CH2CHR) ppm.
2.13. Synthesis of P(GDDAc)27 (9)
To a mixture of P(GHCTL)27 (8) (0.301 g, 1.386 mmol HCTL) and dodecyl acrylate (0.833 g, 3.464 mmol) in chloroform (3.0 mL), 3-(dimethylamino)-1-propylamine (0.353 g, 3.464 mmol) was added in 30 min via syringe pump at room temperature. The mixture was stirred for 20 h at room temperature. Chloroform was removed under reduced pressure. Dialysis in acetone gave 9 as a colorless, viscous liquid (0.628 g, 81%). Mn,NMR = 15,115 g·mol−1, Mn,SEC = 15,300 g·mol−1, Ð = 1.36. 1H NMR (400 MHz, CDCl3) (9): δ = 0.82 (t, 3JH,H = 6.8 Hz, CH2CH2(CH2)9CH3), 1.20 (s, CH2CH2(CH2)9CH3), 1.48–1.69 (m, CH2CH2N(CH3)2, CH2CH2(CH2)9CH3, ArCH2CH2), 1.76–2.08 (m, CHCH2CH2SCH2), 2.15 (s, CH2N(CH3)2), 2.22–2.37 (m, CH2N(CH3)2), 2.43–2.61 (m, CHCH2CH2SCH2CH2), 2.64–2.78 (m, CHCH2CH2SCH2CH2), 3.07–3.37 (m, NHCH2CH2CH2N(CH3)2), 3.41–3.75 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.77–4.07 (m, CH2OC=ONHCH), 4.10–4.41 (m, O=COCH2CH2), 7.09–7.23 (m, Ar), 7.76 (br. s, NH) ppm. 13C NMR (101 MHz, CDCl3) (9): δ = 14.1 (CH2CH2(CH2)9CH3), 22.7–31.9 (CH2CH2(CH2)9CH3, CHCH2CH2SCH2, ArCH2CH2), 26.8 (CHCH2CH2SCH2CH2), 28.2 (CH2CH2N(CH3)2), 28.6 (CHCH2CH2SCH2CH2), 34.7 (CHCH2CH2SCH2CH2), 38.7 (NHCH2CH2CH2N(CH3)2), 45.5 (CH2N(CH3)2), 54.1 (NHCH), 57.9 (CH2N(CH3)2), 64.9 (O=COCH2CH2), 125.9 (ArCH2), 128.3 (ArCH2), 128.5 (ArCH2), 140.0 (ArCH2), 156.4 (OC=ONH), 171.5 (CHC=ONH), 172.0 (CH2C=OO) ppm.
2.14. Synthesis of P(GDDAc, q)27 (10)
P(GDDAc)27 (9) (0.431 g, 0.770 mmol –NMe2) was dissolved in THF (9.0 mL), and methyl iodide (0.131 g, 0.923 mmol) was added. The solution was stirred at room temperature for 20 h. Removal of THF and excess methyl iodide under reduced pressure and dialysis in methanol gave polymer 10 as a slightly yellow solid (0.475 g, 88%). Mn,NMR = 18,947 g·mol−1, Mn,SEC = 11,800 g·mol−1, Ð = 1.36. 1H NMR (400 MHz, CDCl3) (10): δ = 0.81 (t, 3JH,H = 6.5 Hz, CH2CH2(CH2)9CH3), 1.05–1.32 (m, CH2CH2(CH2)9CH3), 1.46–1.62 (m, CH2CH2(CH2)9CH3), 1.85–2.25 (m, CH2CH2N+(CH3)3, ArCH2CH2, CHCH2CH2SCH2), 2.39–2.64 (m, CHCH2CH2SCH2CH2), 2.66–2.82 (m, CHCH2CH2SCH2CH2), 3.05–3.50 (m, NHCH2CH2CH2N+(CH3)3), 3.51–3.83 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.84–4.50 (m, CH2OC=ONHCH, O=COCH2CH2), 7.08–7.24 (m, Ar), 7.77 (br. s, NH) ppm. 13C NMR (101 MHz, CDCl3) (10): δ = 14.1 (CH2CH2(CH2)9CH3), 22.6–31.9 (CH2CH2(CH2)9CH3, CHCH2CH2SCH2, ArCH2CH2), 26.7 (CHCH2CH2SCH2CH2), 28.3 (CH2CH2N+(CH3)3), 28.6 (CHCH2CH2SCH2CH2), 34.8 (CHCH2CH2SCH2CH2), 40.1 (NHCH2CH2CH2N+(CH3)3), 53.8 (CH2N+(CH3)3, NHCH), 64.9 (CH2N+(CH3)3, O=COCH2CH2), 156.5 (OC=ONH), 172.1 (CHC=ONH), 172.8 (CH2C=OO) ppm.
2.15. Synthesis of P(GDMAPA14-co-GDDADDAc13) (11)
P(GNPC)27 (7) (0.541 g, 2.26 mmol carbonate) was dissolved in DMF (11 mL) and 3-(dimethylamino)-1-propylamine (0.115 g, 1.13 mmol) was added in 30 min via syringe pump. The mixture was stirred for 20 h at room temperature. dl-homocysteine thiolactone hydrochloride (0.174 g, 1.13 mmol) and 4-(dimethylamino)pyridine (0.013 g, 0.11 mmol) were added. The mixture was cooled to 0 °C, and triethylamine (0.229 g, 2.26 mmol) was added over 1 h via syringe pump. The solution was stirred for 20 h at room temperature. DMF was removed under reduced pressure at room temperature. The crude product was dissolved in chloroform, and dodecyl acrylate (0.680 g, 2.83 mmol) was added. The mixture was cooled to 0 °C, and dodecylamine (0.525 g, 2.83 mmol) was added in 30 min using a syringe pump. After stirring for 20 h at room temperature, the solvent was removed and the crude product was purified by dialysis in acetone. Polymer 11 was received as a slightly yellow, viscous liquid (0.469 g, 48%). Mn,NMR = 11,631 g·mol−1, Mn,SEC = 10,800 g·mol−1, Ð = 1.36. 1H NMR (400 MHz, CDCl3/acetone-d6 (6:4)) (11): δ = 0.81 (t, 3JH,H = 6.6 Hz CH2CH2(CH2)9CH3), 1.20 (s, CH2CH2(CH2)9CH3), 1.38–1.50 (m, NHCH2CH2(CH2)9CH3), 1.51–1.64 (m, OCH2CH2(CH2)9CH3), 1.70–1.86 (m, CH2CH2N(CH3)2), 1.88–2.01 (m, CHCH2CH2SCH2), 2.29–2.77 (m, CH2N(CH3)2, CHCH2CH2SCH2CH2), 2.97–3.30 (m, NHCH2CH2CH2N(CH3)2, NHCH2CH2(CH2)9CH3), 3.44–3.79 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.90–4.34 (m, CH2OC=ONHR, CH2OC=ONHCH, O=COCH2CH2), 7.07–7.24 (m, Ar) ppm. 13C NMR (101 MHz, CDCl3/acetone-d6 (6:4)) (11): δ = 13.5 (CH2CH2(CH2)9CH3), 22.1–31.4 (CH2CH2(CH2)9CH3, CHCH2CH2SCH2, ArCH2CH2, CH2CH2N(CH3)2), 34.2 (CHCH2CH2SCH2CH2), 38.4 (NHCH2CH2CH2N(CH3)2), 39.0 (NHCH2CH2(CH2)9CH3), 43.7 (CH2N(CH3)2), 53.3 (NHCH), 56.0 (CH2N(CH3)2), 64.2 (O=COCH2CH2), 127.8 (ArCH2), 127.9 (ArCH2), 155.9 (OC=ONH), 156.2 (OC=ONH), 171.4 (CHC=ONH), 172.1 (CH2C=OO) ppm.
2.16. Synthesis of P(GTMAPA14-co-GDDADDAc13) (12)
P(GDMAPA14-co-GDDADDAc13) (11) (0.179 g, 0.20 mmol –NMe2) was dissolved in THF (2.0 mL), and methyl iodide (0.036 g, 0.25 mmol) was added. The solution was stirred at room temperature for 20 h. Removal of THF and excess methyl iodide under reduced pressure and dialysis in methanol gave polymer 12 as a slightly yellow solid (0.203 g, quant.). Mn,NMR = 13,476 g·mol−1, Mn,SEC = 9400 g·mol−1, Ð = 1.41. 1H-NMR (400 MHz, CDCl3/acetone-d6 (6:4)) (12): δ = 0.80 (t, 3JH,H = 6.8 Hz CH2CH2(CH2)9CH3), 1.18 (s, CH2CH2(CH2)9CH3), 1.36–1.48 (m, NHCH2CH2(CH2)9CH3), 1.50–1.66 (m, OCH2CH2(CH2)9CH3), 1.74–2.01 (m, CH2CH2N+(CH3)3, CHCH2CH2SCH2), 2.42–2.89 (m, CHCH2CH2SCH2CH2), 2.94–3.45 (m, NHCH2CH2CH2N+(CH3)3, NHCH2CH2(CH2)9CH3), 3.49–3.81 (m, ArCH2CH2CH2, OCH2CH(CH2OC=ONHR)O), 3.87–4.38 (m, CH2OC=ONHR, CH2OC=ONHCH, O=COCH2CH2), 7.03–7.23 (m, Ar) ppm. 13C NMR (101 MHz, CDCl3/acetone-d6 (6:4)) (12): δ = 13.4 (CH2CH2(CH2)9CH3), 22.1–31.3 (CH2CH2(CH2)9CH3, CHCH2CH2SCH2, ArCH2CH2, CH2CH2N(CH3)2), 34.2 (CHCH2CH2SCH2CH2), 38.9 (NHCH2CH2(CH2)9CH3), 43.0 (NHCH2CH2CH2N+(CH3)2), 53.0 (CH2N+(CH3)3), 53.8 (NHCH), 64.1 (CH2N(CH3)2,2 O=COCH2CH2), 127.8 (ArCH2), 127.9 (ArCH2), 141.3 (ArCH2), 155.9 (OC=ONH), 156.4 (OC=ONH), 171.3 (CHC=ONH), 171.4 (CH2C=OO) ppm.