A New Route for Preparation of Hydrophobic Silica Nanoparticles Using a Mixture of Poly(dimethylsiloxane) and Diethyl Carbonate
Abstract
:1. Introduction
2. Materials and Methods
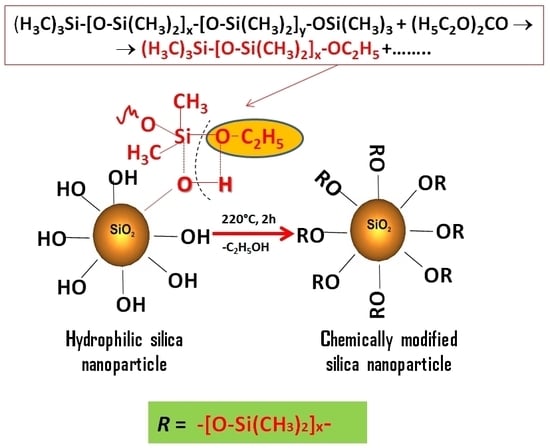
2.1. The Strategy of Silica Surface Modification
2.2. Reagents
2.3. Infrared Spectroscopy
2.4. Contact Angles
2.5. Elemental Analysis
2.6. TEM and AFM
2.7. Rheology of Modified Silica Nanoparticles
3. Results and Discussion
3.1. Infrared Spectroscopy Analysis Results
3.2. Elemental Analysis Results
3.3. Rheology Analysis Results
3.4. AFM and TEM Analysis Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Litvinov, V.M.; Barthel, H.; Weis, J. Structure of a PDMS Layer Grafted onto a Silica Surface Studied by Means of DSC and Solid-State NMR. Macromolecules 2002, 35, 4356–4364. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.; Amalnerkar, D.P.; Seth, T. Surface chemical modification of silica aerogels using various alkyl-alkoxy/chloro silanes. Appl. Surf. Sci. 2003, 206, 262–270. [Google Scholar] [CrossRef]
- Park, S.E.; Prasetyanto, E.A. Morphosynthesis and Catalysis by Organofunctionalized Mesoporous Materials. In Organosilanes, Properties, Performance, and Applications, UK ed.; Wyman, E.B., Skief, M.C., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 101–131. ISBN 978-1-60876-452-5. [Google Scholar]
- Daoud, W.A.; Xin, J.H.; Xiaoming, T. Synthesis and characterization of hydrophobic silica nanocomposites. Appl. Surf. Sci. 2006, 252, 5368–5371. [Google Scholar] [CrossRef]
- Bernardoni, F.; Kouba, M.; Fadeev, A.Y. Effect of curvature on the packing and ordering of organosilane monolayers supported on solids. Chem. Mater. 2008, 20, 382–387. [Google Scholar] [CrossRef]
- Moitra, N.; Ichii, S.; Kamei, T.; Kanamori, K.; Zhu, Y.; Takeda, K.; Nakanishi, K.; Shimada, T. Surface Functionalization of Silica by Si–H Activation of Hydrosilanes. J. Am. Chem. Soc. 2014, 136, 11570–11573. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.Y.; McCarthy, T.J. Self-assembly is not the only reaction possible between alkyltrichlorosilanes and surfaces: Monomolecular and oligomeric covalently attached layers of dichloro- and trichloro alkylsilanes on silicon. Langmuir 2000, 16, 7268–7274. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Binary monolayer mixtures: Modification of nanopores in silicon-supported tris(trimethylsiloxy)silyl monolayers. Langmuir 1999, 15, 7238–7243. [Google Scholar] [CrossRef]
- Li, Y.-F.; Xia, Y.-X.; Xu, D.-P.; Li, G.-L. Surface Reaction of Particulate Silica with Polydimethylsiloxanes. J. Polym. Sci. 1981, 19, 3069–3079. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; Kazakevich, Y.V. Covalently attached monolayers of oligo(dimethylsiloxane)s on silica: A siloxane chemistry approach for surface modification. Langmuir 2002, 18, 2665–2672. [Google Scholar] [CrossRef]
- Graffius, G.; Bernardoni, F.; Fadeev, A.Y. Covalent Functionalization of Silica Surface Using “Inert” Poly(dimethylsiloxanes). Langmuir 2014, 30, 14797–14807. [Google Scholar] [CrossRef] [PubMed]
- Protsak, I.S.; Kuzema, P.O.; Tertykh, V.A.; Bolbukh, Y.M.; Kozakevich, R.B. Thermogravimetric analysis of silicas chemically modified with products of deoligomerization of polydimethylsiloxane. J. Therm. Anal. Calorim. 2015, 121, 547–557. [Google Scholar] [CrossRef]
- Guba, G.Y.; Bogillo, V.I.; Chuiko, A.A. Kinetics and mechanism of the reaction of organosiloxanes with the surface of pyrogenic silica. Theor. Exp. Chem. 1993, 28, 146–150. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, H.; Wirth, M. Chemical Modification of the Surface of Poly(dimethylsiloxane) by Atom-Transfer Radical Polymerization of Acrylamide. Langmuir 2002, 18, 9971–9976. [Google Scholar] [CrossRef]
- Barthel, H.; Nikitina, E. INS and IR study of Intermolecular Interactions at the Fumed Silica-Polydimethylsiloxane Interphase, Part 3. Silica-Siloxane Adsorption Complexes. Silicon Chem. 2004, 1, 261–279. [Google Scholar] [CrossRef]
- Smith, J.S.; Borodin, O.; Smith, G.D.; Kober, E.M. A Molecular Dynamics Simulation and Quantum Chemistry Study of Poly(dimethylsiloxane)-Silica Nanoparticle Interactions. J. Polym. Sci. B 2007, 45, 1599–1615. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Borysenko, M.V.; Pissis, P.; Spanoudaki, A.; Shinyashiki, N.; Sulim, I.Y.; Kulik, T.V.; Palyanytsy, B.B. Polydimethylsiloxane at the interfaces of fumed silica and zirconia/fumed silica. Appl. Surf. Sci. 2007, 253, 7143–7156. [Google Scholar] [CrossRef]
- Sulym, I.Y.; Borysenko, M.V.; Goncharuk, O.V.; Terpilowski, K.; Sternik, D.; Chibowski, E.; Gun’ko, V.M. Structural and hydrophobic–hydrophilic properties of nanosilica/zirconia alone and with adsorbed PDMS. Appl. Surf. Sci. 2011, 258, 270–277. [Google Scholar] [CrossRef]
- Krumpfer, J.W.; McCarthy, T.J. Rediscovering Silicones: “Unreactive” Silicones React with Inorganic Surfaces. Langmuir 2011, 27, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Wadley, M.L.; Vogt, B.D.; Cavicchi, K.A. Facile Non-Lithographic Route to Highly Aligned Silica Nanopatterns Using Unidirectionally Aligned Polystryrene-Block-Polydimethysiloxane Films. J. Polym. Sci. B 2015, 53, 1058–1064. [Google Scholar] [CrossRef]
- Palacios-Pineda, L.M.; Perales-Martinez, I.A.; Lozano-Sanchez, L.M.; Martínez-Romero, O.; Puente-Córdova, J.; Segura-Cárdenas, E.; Elías-Zúñiga, A. Experimental Investigation of the Magnetorheological Behavior of PDMS Elastomer Reinforced with Iron Micro/Nanoparticles. Polymers 2017, 9, 696. [Google Scholar] [CrossRef]
- Aristova, V.G.; Zimmer, I.M.; Gorbunov, A.I.; Zhilenkov, I.V.; Saushkin, V.V. Mechanism of Siloxane Bond Splitting on a Hydrated Aerosil Surface. Dokl. Akad. Nauk SSSR 1980, 255, 131–134. [Google Scholar]
- Tertykh, V.A.; Pavlov, V.V. Reactivity of molecules attacking a functional center. Adsorpt. Adsorbt. Duide 1978, 6, 67–75. [Google Scholar]
- Pavlov, V.V.; Guba, G.Y.; Tertykh, V.A.; Chuiko, A.A. Study of the interaction of alkylsiloxanes with the surface of dispersed silicas. Adsorpt. Adsorbt. Duide 1980, 8, 35–39. [Google Scholar]
- Chang, C.L.; Lee, H.S.; Chen, C.K. Aminolysis of cured siloxane polymers. Polym. Degrad. Stab. 1999, 65, 1–4. [Google Scholar] [CrossRef]
- Thomas, T.H.; Kendrick, T.C. Thermal Analysis of Polydimethylsiloxanes. I. Thermal Degradation in Controlled Atmospheres. J. Polym. Sci. B 1969, 7, 537–549. [Google Scholar] [CrossRef]
- Clarson, S.J.; Semlyen, J.A. Studies of Cyclic and Linear Poly(dimethylsiloxanes): 21. High Temperature Thermal Behavior. Polymer 1986, 27, 91–95. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Hill, L.W.; Pappas, S.P. Reversible amine solubilization of cured siloxane polymers. J. Appl. Polym. Sci. 1975, 19, 2817–2820. [Google Scholar] [CrossRef]
- Brook, M.A.; Zhao, S.; Liu, L.; Chen, Y. Surface etching of silicone elastomers by depolymerization. Can. J. Chem. 2012, 90, 153–160. [Google Scholar] [CrossRef]
- Qiang, Z.; Gurkan, B.; Ma, J.; Liu, X.; Guo, Y.; Cakmak, M.; Cavicchi, K.A.; Vogt, B.D. Roll-to-roll fabrication of high surface area mesoporous carbon with process-tunable pore texture for optimization of adsorption capacity of bulky organic dyes. Microporous Mesoporous Mater. 2016, 227, 57–64. [Google Scholar] [CrossRef]
- Selva, M.; Fabrisa, M.; Perosa, A. Decarboxylation of dialkyl carbonates to dialkyl ethers over alkali metal-exchanged faujasites. Green Chem. 2011, 13, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Tundo, P.; Perosa, A.; Zecchini, F. Methods and Reagents for Green Chemistry: An Introduction, 1st ed.; Willey: Venezia, Italy, 2007; 336p, ISBN 978-0-471-75400-8. [Google Scholar]
- Ono, Y.; Akiyama, M.; Suzuki, E. Direct Synthesis of Tetraalkoxysilanes from Silica by Reaction with Dialkyl Carbonates. Chem. Mater. 1993, 5, 442–447. [Google Scholar] [CrossRef]
- Okamoto, M.; Suzuki, S.; Suzuki, E. Polysiloxane depolymerization with dimethyl carbonate using alkali metal halide catalysts. Appl. Catal. A 2004, 261, 239–245. [Google Scholar] [CrossRef]
- Demianenko, E.M.; Grebenyuk, A.G.; Lobanov, V.V.; Protsak, I.S.; Kozakevych, R.B.; Bolbukh, Y.M.; Tertykh, V.A. Quantum chemical study on interaction of dimethyl carbonate with polydimethylsiloxane. Chem. Phys. Technol. Surf. 2014, 5, 473–479. [Google Scholar]
- Protsak, I.S.; Tertykh, V.A.; Pakhlov, E.M.; Derylo-Marczewska, A. Modification of fumed silica surface with mixtures of polyorganosiloxanes and dialkyl carbonates. Prog. Org. Coat. 2017, 106, 163–169. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Pakhlov, E.M.; Goncharuk, O.V.; Andriyko, L.S.; Marynin, A.I.; Ukrainets, A.I.; Charmas, B.; Skubiszewska-Zieba, J.; Blitz, J.P. Influence of hydrophobization of fumed oxides on interactions with polar and nonpolar adsorbates. Appl. Surf. Sci. 2017, 423, 855–868. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V. Nuclear Magnetic Resonance Studies of Interfacial Phenomena (Surfactant Science), 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; 1040p, ISBN 978-1466551688. [Google Scholar]
- Legrand, A.P. (Ed.) The Surface Properties of Silicas, 1st ed.; Wiley: New York, NY, USA, 1998; 494p, ISBN 978-0471953326. [Google Scholar]
- Kiselev, A.V.; Lygin, V.I. Infrared Spectra of Surface Compounds, 1st ed.; Wiley: New York, NY, USA, 1975; 384p, ISBN 978-0470489055. [Google Scholar]
- Hair, M.L. Infrared Spectroscopy in Surface Chemistry, 1st ed.; Dekker: New York, NY, USA, 1967; 336p, ISBN 978-0824712853. [Google Scholar]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protsak, I.; Pakhlov, E.; Tertykh, V.; Le, Z.-C.; Dong, W. A New Route for Preparation of Hydrophobic Silica Nanoparticles Using a Mixture of Poly(dimethylsiloxane) and Diethyl Carbonate. Polymers 2018, 10, 116. https://doi.org/10.3390/polym10020116
Protsak I, Pakhlov E, Tertykh V, Le Z-C, Dong W. A New Route for Preparation of Hydrophobic Silica Nanoparticles Using a Mixture of Poly(dimethylsiloxane) and Diethyl Carbonate. Polymers. 2018; 10(2):116. https://doi.org/10.3390/polym10020116
Chicago/Turabian StyleProtsak, Iryna, Evgeniy Pakhlov, Valentyn Tertykh, Zi-Chun Le, and Wen Dong. 2018. "A New Route for Preparation of Hydrophobic Silica Nanoparticles Using a Mixture of Poly(dimethylsiloxane) and Diethyl Carbonate" Polymers 10, no. 2: 116. https://doi.org/10.3390/polym10020116
APA StyleProtsak, I., Pakhlov, E., Tertykh, V., Le, Z. -C., & Dong, W. (2018). A New Route for Preparation of Hydrophobic Silica Nanoparticles Using a Mixture of Poly(dimethylsiloxane) and Diethyl Carbonate. Polymers, 10(2), 116. https://doi.org/10.3390/polym10020116