α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Soluble α-CD Polymer
2.2. Synthesis of Insoluble α-CD Nanosponge
2.3. Preparation of α-CD-based Formulations
2.4. Characterization of α-CD-Based Formulations
2.5. Hemolytic Assay
2.6. In Vitro Oxygen Release Determination
2.7. Cell Culture
2.8. Stability Determination of α-CD-based Formulations in Cell Culture Medium and Ischemic Buffer
2.9. Protocols
2.9.1. Normoxic Experimental Conditions (Dose–Response Studies)
- Oxygenated α-CD POLY Groups (O-α-CD POLY) and Nitrogen α-CD POLY Groups (N-α-CD POLY)
- Oxygenated α-CD NS Groups (O-α-CD NS) and Nitrogen α-CD NS Groups (N-α-CD NS)
- Oxygenated α-CD Groups (O-α-CD) and Nitrogen α-CD Groups (N-α-CD)
2.9.2. Hypoxia/Reoxygenation Experimental Conditions
2.9.3. Pre-Treatment with α-CD-Based Formulations
2.9.4. Post-Treatment with α-CD-Based Formulations
2.10. MTT Assay
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characterisation of α-CD-Based Formulations
3.2. Evaluation of α-CD-Based Formulation Biocompatibility
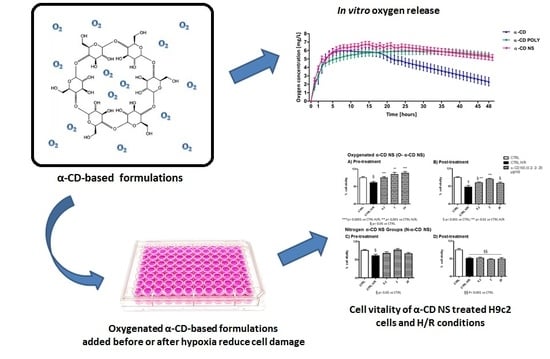
3.3. In Vitro Oxygen Release Kinetics
3.4. Stability Determination of α-CD-Based Formulations in Cell Culture Medium and Ischemic Buffer
3.5. Dose–Response in Normoxic Conditions
3.6. Untreated Cells: Normoxic and H/R Conditions
3.7. NS-Treated Cells and H/R Conditions
3.7.1. α-CD POLY
3.7.2. α-CD NS
3.7.3. α-CD
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, P.; Penna, C. Redox signaling and cardioprotection: Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 1974–1995. [Google Scholar] [CrossRef] [PubMed]
- Tullio, F.; Angotti, C.; Perrelli, M.G.; Penna, C.; Pagliaro, P. Redox balance, and cardioprotection. Basic Res. Cardiol. 2013, 108, 392. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Granata, R.; Gabriele Tocchetti, C.; Pia Gallo, M.; Alloatti, G.; Pagliaro, P. Endogenous cardioprotective agents: Role in pre and postconditioning. Curr. Drug Targets 2015, 16, 843–867. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.W.; Iyer, K.S.; Hool, L.C. The potential for nanotechnology to improve delivery of therapy to the acute ischemic heart. Nanomedicine 2016, 11, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Bassino, E.; Alloatti, G. Platelet activating factor: The good and the bad in the ischemic/reperfused heart. Exp. Biol. Med. (Maywood) 2011, 236, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.; Condorelli, G.; Davidson, S.M.; Engel, F.B.; Ferdinandy, P.; Hausenloy, D.J.; Lecour, S.; Madonna, R.; Ovize, M.; Ruiz-Meana, M.; et al. Novel therapeutic strategies for cardioprotection. Pharmacol. Ther. 2014, 144, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Di Venosa, N.; Ruggiero, F.M.; Pistolese, M.; D’Agostino, D.; Tiravanti, E.; Fiore, T.; Paradies, G. Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim. Biophys. Acta 2005, 1710, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Rastaldo, R.; Mancardi, D.; Raimondo, S.; Cappello, S.; Gattullo, D.; Losano, G.; Pagliaro, P. Post–conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP–sensitive K+ channel and protein kinase C activation. Basic Res. Cardiol. 2006, 101, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.M.; Yokoyama, T.; Horikawa, Y.; Roth, D.M.; Patel, H.H. Reactive oxygen species trigger ischemic and pharmacological postconditioning: In vivo and in vitro characterization. Life Sci. 2007, 81, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J.; Granfeldt, A.; Mykytenko, J.; Undyala, V.V.; Dong, Y.; Przyklenk, K. The multidimensional physiological responses to postconditioning. Antioxid. Redox Signal. 2011, 14, 791–810. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Kulkarni, Y.A.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and repeated dose toxicity studies of different β-cyclodextrin-based nanosponge formulations. J. Pharm. Sci. 2015, 104, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Torne, S.J.; Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010, 17, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, C.L.; Ferrara, B.; Occhipinti, S.; Boggio, E.; Barrera, G.; Pizzimenti, S.; Giovarelli, M.; Fantozzi, R.; Chiocchetti, A.; Argenziano, M.; et al. Enhanced cytotoxic effect of camptothecin nanosponges in anaplastic thyroid cancer cells in vitro and in vivo on orthotopic xenograft tumors. Drug Deliv. 2017, 24, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Leeves, N.; Bjornsdottir, B.; Duffy, L.; Masson, M. Effect of cyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo. J. Pharm. Sci. 1999, 88, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Puskás, I.; Pápai, K.; Salvador, E.; Roewer, N.; Förster, C.; Broscheit, J.A. Sevoflurane-sulfobutylether-β-cyclodextrin complex: Preparation, characterization, cellular toxicity, molecular modeling and blood-brain barrier transport studies. Molecules 2015, 20, 10264–10279. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Salmas, R.E.; Salvador, E.; Roewer, N.; Broscheit, J.; Förster, C. Evaluation of the potential toxicity of unmodified and modified cyclodextrins on murine blood-brain barrier endothelial cells. J. Toxic. Sci. 2016, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Akhter, A.K.; Bisazza, A.; Giustetto, P.; Trotta, F.; Vavia, P. Nanosponge formulations as oxygen delivery systems. Int. J. Pharm. 2010, 402, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Caldera, F.; Cavalli, R.; Mele, A.; Punta, C.; Melone, L.; Castiglione, F.; Rossi, B.; Ferro, M.; Crupi, V.; et al. Synthesis and characterization of a hyper-branched water-soluble β-cyclodextrin polymer. Beilstein J. Org. Chem. 2014, 10, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Tumiatti, W. Cross-Linked Polymers Based on Cyclodextrins for Removing Polluting Agents. WO 03/085002 A1, 16 October 2003. [Google Scholar]
- Pasqua, T.; Tota, B.; Penna, C.; Corti, A.; Cerra, M.C.; Loh, Y.P.; Angelone, T. pGlu-serpinin protects the normotensive and hypertensive heart from ischemic injury. J. Endocrinol 2015, 227, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guan, Y.; Duan, J.; Wei, G.; Zhu, Y.; Quan, W.; Guo, C.; Zhou, D.; Wang, Y.; Xi, M.; et al. Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur. J. Pharmacol. 2013, 699, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Perrelli, M.G.; Karam, J.P.; Angotti, C.; Muscari, C.; Montero-Menei, C.N.; Pagliaro, P. Pharmacologically active microcarriers influence VEGF-A effects on mesenchymal stem cell survival. J. Cell. Mol. Med. 2013, 17, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motoyama, K.; Arima, H.; Toyodome, H.; Irie, T.; Hirayama, F.; Uekama, K. Effect of 2,6-di-O-methyl-alpha-cyclodextrin on hemolysis and morphological change in rabbit’s red blood cells. Eur. J. Pharm. Sci. 2006, 29, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; García-González, C.A.; Concheiro, A. Cyclodextrins as versatile building blocks for regenerative medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Giustetto, P.; Rolfo, A.; Caniggia, I.; Balbis, S.; Guiot, C.; Cavalli, R. Microbubble-mediated oxygen delivery to hypoxic tissues as a new therapeutic device. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society EMBS 2008, Vancouver, BC, Canada, 20–25 August 2008; pp. 2067–2070. [Google Scholar]
- Cavalli, R.; Bisazza, A.; Giustetto, P.; Civra, A.; Lembo, D.; Trotta, G.; Guiot, C.; Trotta, M. Preparation and characterization of dextran nanobubbles for oxygen delivery. Int. J. Pharm. 2009, 381, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bisazza, A.; Rolfo, A.; Balbis, S.; Madonnaripa, D.; Caniggia, I.; Guiot, C. Ultrasound-mediated oxygen delivery from chitosan nanobubbles. Int. J. Pharm. 2009, 378, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Magnetto, C.; Prato, M.; Khadjavi, A.; Giribaldi, G.; Fenoglio, I.; Jose Gulino, G.; Cavallo, F.; Quaglino, E.; Benintende, E.; Varetto, G.; et al. Ultrasound-activated decafluoropentane-cored and chitosan-shelled nanodroplets for oxygen delivery to hypoxic cutaneous tissues. RCS Adv. 2014, 4, 38433–38441. [Google Scholar] [CrossRef]
- Prato, M.; Magnetto, C.; Jose, J.; Khadjavi, A.; Cavallo, F.; Quaglino, E.; Panariti, A.; Rivolta, I.; Benintende, E.; Varetto, G.; et al. 2H, 3H-decafluoropentane-based nanodroplets: New perspectives for oxygen delivery to hypoxic cutaneous tissues. PLoS ONE 2015, 10, e0119769. [Google Scholar] [CrossRef] [PubMed]
- Cramer, F.; Henglein, F.M. Einschlussverbindungen der cyclodextrine mit gasen. Angew. Chem. 1956, 68, 649. [Google Scholar] [CrossRef]
- Trotta, F.; Cavalli, R.; Martina, K.; Biasizzo, M.; Vitillo, J.; Bordiga, S.; Vavia, P.; Ansari, K. Cyclodextrin nanosponges as effective gas carriers. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 189–194. [Google Scholar] [CrossRef]
- Dufort, S.; Sancey, L.; Coll, J.L. Physico-chemical parameters that govern nanoparticles fate also dictate rules for their molecular evolution. Adv. Drug Deliv. Rev. 2012, 64, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; Ullio, C.; Argenziano, M.; Dianzani, C.; Cavalli, R.; Trotta, F.; Ferretti, C.; Zara, G.P.; Gigliotti, L.C.; Ciamporcero, E.; et al. GSH-targeted nanosponges increase doxorubicin-induced toxicity “in vitro” and “in vivo” in cancer cells with high antioxidant defenses. Free Radic. Biol. Med. 2016, 97, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.P.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef] [PubMed]









| Formulation | Average diameter ± SD (nm) | PDI * | Z-potential ± SD (mV) |
|---|---|---|---|
| α-CD ** | - | - | −29.12 ± 4.74 |
| α-CD POLY ** | - | - | −12.87 ± 4.60 |
| α-CD NS | 850.55 ± 57.90 | 0.17 | −36.37 ± 3.58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Femminò, S.; Penna, C.; Bessone, F.; Caldera, F.; Dhakar, N.; Cau, D.; Pagliaro, P.; Cavalli, R.; Trotta, F. α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers 2018, 10, 211. https://doi.org/10.3390/polym10020211
Femminò S, Penna C, Bessone F, Caldera F, Dhakar N, Cau D, Pagliaro P, Cavalli R, Trotta F. α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers. 2018; 10(2):211. https://doi.org/10.3390/polym10020211
Chicago/Turabian StyleFemminò, Saveria, Claudia Penna, Federica Bessone, Fabrizio Caldera, Nilesh Dhakar, Daniele Cau, Pasquale Pagliaro, Roberta Cavalli, and Francesco Trotta. 2018. "α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model" Polymers 10, no. 2: 211. https://doi.org/10.3390/polym10020211
APA StyleFemminò, S., Penna, C., Bessone, F., Caldera, F., Dhakar, N., Cau, D., Pagliaro, P., Cavalli, R., & Trotta, F. (2018). α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers, 10(2), 211. https://doi.org/10.3390/polym10020211