Structural Investigation of Poly(ethylene furanoate) Polymorphs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Diffraction and Infrared Measurements
2.3. Structure Determinations
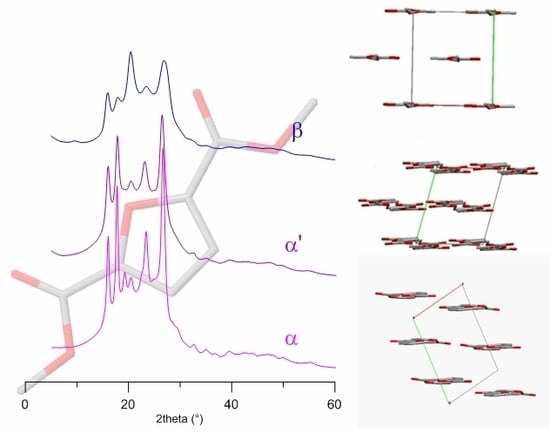
3. Results and Discussion
3.1. Sample Characterization
3.2. Crystal Solutions and Refinements
3.3. α-PEF
3.4. α’-PEF
3.5. β-PEF
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- European Bioplastics, Bioplastics Facts and Figures. Available online: http://www.european-bioplastics.org/news/publications/ (accessed on 31 October 2017).
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M.W. A scalable carboxylation route to furan-2,5-dicarboxylic acid. Green Chem. 2017, 19, 2966–2972. [Google Scholar] [CrossRef]
- "Who we are" page of Synvina web site. Available online: https:/www.synvina.com/about-us/who-we-are/ (accessed on 8 March 2018).
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon Dioxide Sorption and Transport in Amorphous Poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaija, A.P.C.; Patela, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance Energy. Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Guigo, N.; van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Modelling the non-isothermal crystallization of polymers: Application to poly(ethylene 2,5-furandicarboxylate). Thermochim. Acta 2017, 650, 66–75. [Google Scholar] [CrossRef]
- Dimitriadis, T.; Bikiaris, D.N.; Papageorgiou, G.Z.; Floudas, G. Molecular Dynamics of Poly(ethylene-2,5-furanoate) (PEF) as a Function of the Degree of Crystallinity by Dielectric Spectroscopy and Calorimetry. Macromol. Chem. Phys. 2016, 217, 2056–2062. [Google Scholar] [CrossRef]
- Stoclet, G.; Gobius du Sart, G.; Yeniad, B.; de Vos, S.; Lefebvre, J.M. Isothermal crystallization and structural characterization of poly(ethylene-2,5-furanoate). Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and Polymorphism of Poly(ethylene furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Nadia Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and structural response of in situ prepared biobased poly(ethylene 2,5-furan dicarboxylate) nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Kazaryan, L.G.; Medvedeva, F.M. X-ray study of poly(ethylene furan-2,5-dicarboxylate) structure. Vysokomol. Soedin. Ser. B Kratk. Soobshcheniya 1968, 10, 305–306. [Google Scholar]
- Mao, Y.; Kriegel, R.M.; Bucknall, D.G. The crystal structure of poly(ethylene furanoate). Polymer 2016, 102, 308–314. [Google Scholar] [CrossRef]
- Stoclet, G.; Lefebvre, J.M.; Yeniad, B.; Gobius du Sart, G.; de Vos, S. On the strain-induced structural evolution of Poly(ethylene-2,5-furanoate) upon uniaxial stretching: An in-situ SAXS-WAXS study. Polymer 2018, 134, 227–241. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: Thermal behavior comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Tse, J.S.; Mak, T.C.W. Refinement of the crystal structure of polyethylene terephthalate. J. Cryst. Mol. Struct. 1975, 5, 75–80. [Google Scholar] [CrossRef]






| Cell Parameters | α-PEF | α’-PEF | β-PEF |
|---|---|---|---|
| a (Å) | 5.729(7) | 5.912(3) | 5.953(3) |
| b (Å) | 7.89(3) | 6.913(4) | 6.600(3) |
| c (Å) | 9.62(6) | 19.73(2) | 10.52(1) |
| α (°) | 98.1(3) | 90.0 | 90.0 |
| β (°) | 65.1(4) | 90.0 | 107.0(1) |
| γ (°) | 101.3(4) | 104.41(3) | 90.0 |
| S.G. | P-1 | P1 | P1 |
| Vol (Å3) | 385.85 | 780.84 | 394.30 |
| ρcalc (g/cm3) | 1.567 | 1.549 | 1.482 |
| Rp (%) | 12.5 | 11.4 | 13.2 |
| Rwp (%) | 13.2 | 12.1 | 13.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maini, L.; Gigli, M.; Gazzano, M.; Lotti, N.; Bikiaris, D.N.; Papageorgiou, G.Z. Structural Investigation of Poly(ethylene furanoate) Polymorphs. Polymers 2018, 10, 296. https://doi.org/10.3390/polym10030296
Maini L, Gigli M, Gazzano M, Lotti N, Bikiaris DN, Papageorgiou GZ. Structural Investigation of Poly(ethylene furanoate) Polymorphs. Polymers. 2018; 10(3):296. https://doi.org/10.3390/polym10030296
Chicago/Turabian StyleMaini, Lucia, Matteo Gigli, Massimo Gazzano, Nadia Lotti, Dimitrios N. Bikiaris, and George Z. Papageorgiou. 2018. "Structural Investigation of Poly(ethylene furanoate) Polymorphs" Polymers 10, no. 3: 296. https://doi.org/10.3390/polym10030296
APA StyleMaini, L., Gigli, M., Gazzano, M., Lotti, N., Bikiaris, D. N., & Papageorgiou, G. Z. (2018). Structural Investigation of Poly(ethylene furanoate) Polymorphs. Polymers, 10(3), 296. https://doi.org/10.3390/polym10030296