Ultrasound- and Thermo-Responsive Ionic Liquid Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Sodium 2-Acrylamido-2-Methylpropanesulfonate) (PAMPSNa)
2.3. Synthesis of Poly(Tributyl-N-Octylphosphonium 2-Acrylamido-2-Methylpropanesulfonate) (PAMPSP4448)
2.4. Synthesis of Poly(Tetrabutylphosphonium 2-Acrylamido-2-Methylpropanesulfonate) (PAMPSP4444)
2.5. Preparation of Aqueous Polymer Solution
2.6. Measurements
3. Results and Discussion
3.1. Synthesis of Polymers
3.2. Turbidimetry
3.3. Light Scattering
3.4. Fluorescence Probe
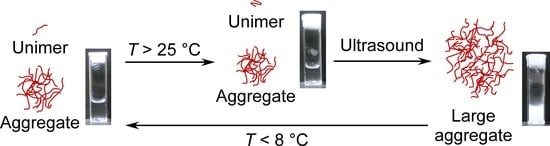
3.5. The Proposed Mechanism of Ultrasound- and Thermo-Responsive Behavior
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takahashi, H.; Matsuzaka, N.; Nakayama, M.; Kikuchi, A.; Yamato, M.; Okano, T. Terminally functionalized thermoresponsive polymer brushes for simultaneously promoting cell adhesion and cell sheet harvest. Biomacromolecules 2012, 13, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, H. Stimuli-responsive supramolecular polymers in aqueous solution. Acc. Chem. Res. 2014, 47, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, D.; Jiang, Y.; Laaser, J.E.; Lodge, T.P.; Reineke, T.M. Tuning cationic block copolymer micelle size by pH and ionic strength. Biomacromolecules 2016, 17, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Ghazwani, M.; Zhang, P.; Li, J.; Thorne, S.H.; Li, S. Tunable pH-responsive polymeric micelle for cancer treatment. ACS Macro Lett. 2015, 4, 620–623. [Google Scholar] [CrossRef]
- Murata, H.; Cummings, C.S.; Koepsel, R.R.; Russell, A.J. Polymer-based protein engineering can rationally tune enzyme activity, pH-dependence, and stability. Biomacromolecules 2013, 14, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Suthar, K.; Mancini, D.C. Role of solvation dynamics and local ordering of water in inducing conformational transitions in poly(N-isopropylacrylamide) oligomers through the LCST. J. Phys. Chem. B 2012, 116, 2651–2663. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shen, L.; Wu, C. LLS and FTIR studies on the hysteresis in association and dissociation of poly(N-isopropylacrylamide) chains in water. Macromolecules 2006, 39, 2325–2329. [Google Scholar] [CrossRef]
- Kohno, Y.; Saita, S.; Men, Y.; Yuan, J.; Ohno, H. Thermoresponsive polyelectrolytes derived from ionic liquids. Polym. Chem. 2015, 6, 2163–2178. [Google Scholar] [CrossRef]
- Deguchi, Y.; Kohno, Y.; Ohno, H. A fine tuning of LCST-type phase transition of poly(ionic liquid)s in water. Chem. Lett. 2015, 44, 238–240. [Google Scholar] [CrossRef]
- Vchirawongkwin, V.; Pornpiganon, C.; Kritayakornupong, C.; Tongraar, A.; Rode, B.M. The stability of bisulfite and sulfonate ions in aqueous solution characterized by hydration structure and dynamics. J. Phys. Chem. B. 2012, 116, 11498–11507. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Schlaad, H.; Yuan, J. Cationic poly(ionic liquid) with tunable lower critical solution temperature-type phase transition. ACS Macro Lett. 2013, 2, 456–459. [Google Scholar] [CrossRef]
- Seshadri, R.; Weiss, J.; Hulbert, G.J.; Mount, J. Ultrasonic processing influences rheological and optical properties of high-methoxyl pectin dispersions. Food Hydrocoll. 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Massey, J.; Power, K.N.; Manners, I.; Winnik, M.A. Self-assembly of a novel organometallic−inorganic block copolymer in solution and the solid state: Nonintrusive observation of novel wormlike poly(ferrocenyldimethylsilane)-b-poly(dimethylsiloxane) micelles. J. Am. Chem. Soc. 1998, 120, 9533–9540. [Google Scholar] [CrossRef]
- Wang, J.; Pelletier, M.; Zhang, H.; Xia, H.; Zhao, Y. High-frequency ultrasound-responsive block copolymer micelle. Langmuir 2009, 25, 13201–13205. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Boissière, O.; Zhao, Y.; Yan, B.; Tremblay, L.; Lacelle, S.; Xia, H.; Zhao, Y. Ultrasound-responsive block copolymer micelles based on a new amplification mechanism. Langmuir 2012, 28, 16463–16468. [Google Scholar] [CrossRef] [PubMed]
- Naota, T.; Koori, H. Molecules that assemble by sound: An application to the instant gelation of stable organic fluids. J. Am. Chem. Soc. 2005, 127, 9324–9325. [Google Scholar] [CrossRef] [PubMed]
- Komiya, N.; Muraoka, T.; Iida, M.; Miyanaga, M.; Takahashi, K.; Naota, T. Ultrasound-induced emission enhancement based on structure-dependent homo- and heterochiral aggregations of chiral binuclear platinum complexes. J. Am. Chem. Soc. 2011, 133, 16054–16061. [Google Scholar] [CrossRef] [PubMed]
- Mitsukami, Y.; Donovan, M.S.; Lowe, A.B.; McCormick, C.L. Water-soluble polymers. 81. Direct synthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via RAFT. Macromolecules 2001, 34, 2248–2256. [Google Scholar] [CrossRef]
- Yusa, S.; Shimada, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. pH-responsive micellization of amphiphilic diblock copolymers synthesized via reversible addition-fragmentation chain transfer polymerization. Macromolecules 2003, 36, 4208–4215. [Google Scholar] [CrossRef]
- Dubois, E.; Cabuil, V.; Boué, F.; Perzynski, R. Structural analogy between aqueous and oily magnetic fluids. J. Chem. Phys. 1999, 111, 7147–7160. [Google Scholar] [CrossRef]
- Waris, R.; Acree, W.E., Jr.; Street, K.W., Jr. Py and BPe solvent polarity scales: Effect of temperature on pyrene and benzo[ghi]perylene fluorescence spectra. Analyst 1988, 113, 1465–1467. [Google Scholar] [CrossRef] [PubMed]
- Shiratani, M.; Watanabe, Y. Development of photon-counting laser-light-scattering method for detection of nano-particles formed in CVD plasmas. Rev. Laser Eng. 1998, 26, 449–452. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y. Rapid release of entrapped contents from multi-functionalizable, surface cross-linked micelles upon different stimulation. J. Am. Chem. Soc. 2010, 132, 10642–10644. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]








| Polymers | Mn (theory) × 104 | Mn (NMR) × 104 | Mn (GPC) × 104 | Mw/Mn | ER d (%) |
|---|---|---|---|---|---|
| PAMPSNa | 2.11 | 2.09 | 1.85 a | 1.18 a | - |
| PAMPSP4448 | 4.73 b | 4.68 b | 0.58 c | 1.17 c | 95.6 |
| PAMPSP4444 | 3.75 b | 3.75 b | 0.36 c | 1.29 c | 99.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itsuki, K.; Kawata, Y.; Sharker, K.K.; Yusa, S.-i. Ultrasound- and Thermo-Responsive Ionic Liquid Polymers. Polymers 2018, 10, 301. https://doi.org/10.3390/polym10030301
Itsuki K, Kawata Y, Sharker KK, Yusa S-i. Ultrasound- and Thermo-Responsive Ionic Liquid Polymers. Polymers. 2018; 10(3):301. https://doi.org/10.3390/polym10030301
Chicago/Turabian StyleItsuki, Kohei, Yuuki Kawata, Komol Kanta Sharker, and Shin-ichi Yusa. 2018. "Ultrasound- and Thermo-Responsive Ionic Liquid Polymers" Polymers 10, no. 3: 301. https://doi.org/10.3390/polym10030301
APA StyleItsuki, K., Kawata, Y., Sharker, K. K., & Yusa, S.-i. (2018). Ultrasound- and Thermo-Responsive Ionic Liquid Polymers. Polymers, 10(3), 301. https://doi.org/10.3390/polym10030301