Donor-Acceptor-Type Copolymers Based on 3,4-Propylenedioxy-thiophene and 5,6-Difluorobenzotriazole: Synthesis and Electrochromic Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterizations
2.3. Synthesis Process
2.3.1. Synthesis of ProDOT-decyl2
2.3.2. Synthesis of M1
2.3.3. Synthesis of P1, P2 and P3
3. Results and Discussion
3.1. FT-IR Spectra
3.2. Electrochemical Characterization
3.3. Morphology
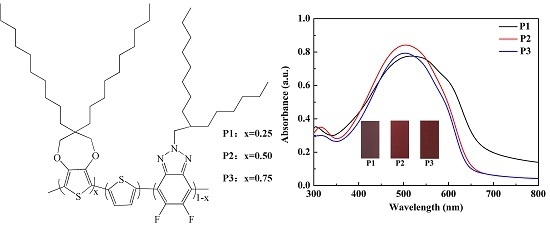
3.4. Optical Properties of Polymer Solutions and Films
3.5. Spectroelectrochemical Properties
3.6. Electrochromic Switching Studies
3.7. Colorimetry
3.8. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Audenaert, M.; Gusman, G.; Deltour, R. Electrical conductivity of I2-doped polyacetylene. Phys. Rev. B 1981, 24, 7380–7382. [Google Scholar] [CrossRef]
- Chiang, C.K.; Gau, S.C.; Fincher, C.R.; Park, Y.W.; MacDiarmid, A.G.; Heeger, A.J. Polyacetylene, (CH)x: n-type and p-type doping and compensation. Appl. Phys. Lett. 1978, 33, 18–20. [Google Scholar] [CrossRef]
- Ustamehmetoğlu, B.; Osken, I.; Cinar, M.E.; Sezer, E.; Karaca, E.; Ozturk, T. Synthesis and Characterization of Dithienothiophene, Bithiazole and Thiophene Containing Polymer. Electrochim. Acta 2017, 227, 435–446. [Google Scholar] [CrossRef]
- Fan, M.J.; Duan, L.R.; Zhou, Y.H.; Wen, S.G.; Li, F.; Liu, D.Y.; Sun, M.L.; Yang, R.Q. Rhodanine side-chained thiophene and indacenodithiophene copolymer for solar cell applications. Mater. Today Energy 2017, 5, 287–292. [Google Scholar] [CrossRef]
- Ramkumar, V.; Kannan, P. Thiophene and furan containing pyrazoline luminescent materials for optoelectronics. J. Lumin. 2016, 169, 204–215. [Google Scholar] [CrossRef]
- ElNahrawy, A.M.; Haroun, A.A.; Hamadneh, I.; Al-Dujaili, A.H.; Kamel, S. Conducting cellulose/TiO2 composites by in situ polymerization of pyrrole. Carbohyd. Polym. 2017, 168, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Li, Y.X.; Yu, X.F.; Yang, Q.B.; Noh, C.H. Using room temperature ionic liquid to fabricate PEDOT/TiO2 nanocomposite electrode-based electrochromic devices with enhanced long-term stability. Sol. Energy Mater. Sol. Cells 2008, 92, 1253–1259. [Google Scholar] [CrossRef]
- Metsik, J.; Timusk, M.; Käämbre, T.; Mändar, H.; Umalas, M.; Kuus, A.; Puust, L.; Utt, K.; Sildos, I.; Mäeorg, U. Stability of poly(3,4-ethylenedioxythiophene) thin films prepared by vapor phase polymerization. Polym. Degrad. Stab. 2016, 126, 170–178. [Google Scholar] [CrossRef]
- Li, H.G.; Xiao, Y.M.; Han, G.Y.; Hou, W.J. Honeycomb-like poly(3,4-ethylenedioxythiophene) as an effective and transparent counter electrode in bifacial dye-sensitized solar cells. J. Power Sources 2017, 342, 709–716. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, W.J.; Park, S.H.; Cho, S.O.; Lee, J.K.; Park, J.B.; Ha, J.S.; Chung, T.H.; Jeong, T. Light-emitting diodes fabricated on an electrical conducting flexible substrate. Solid-State Electron. 2017, 127, 57–60. [Google Scholar] [CrossRef]
- Tian, Y.L.; Zhang, X.; Dou, S.L.; Zhang, L.P.; Zhang, H.M.; Lv, H.M.; Wang, L.L.; Zhao, J.P.; Li, Y. A comprehensive study of electrochromic device with variable infrared emissivity based on polyaniline conducting polymer. Sol. Energy Mater. Sol. Cells 2017, 170, 120–126. [Google Scholar] [CrossRef]
- Kao, S.Y.; Kung, C.W.; Chen, H.W.; Hu, C.W.; Ho, K.C. An electrochromic device based on all-in-one polymer gel through in-situ thermal polymerization. Sol. Energy Mater. Sol. Cells 2016, 145, 61–68. [Google Scholar] [CrossRef]
- Kao, S.Y.; Lin, Y.S.; Hu, C.W.; Leung, M.K.; Ho, K.C. Application of triphenylamine dendritic polymer in a complementary electrochromic device with panchromatic absorption. Sol. Energy Mater. Sol. Cells 2015, 143, 174–182. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, M.; Zhang, Y.; Zhao, J.S.; Fu, C.G. A new electrochromic copolymer which switched between neutral black and oxidized transmissive. RSC Adv. 2016, 6, 80002–80010. [Google Scholar] [CrossRef]
- Xu, Z.P.; Chen, X.M.; Mi, S.; Zheng, J.M.; Xu, C.Y. Solution-processable electrochromic red-to-transmissive polymers with tunable neutral state colors, high contrast and enhanced stability. Org. Electron. 2015, 26, 129–136. [Google Scholar] [CrossRef]
- Ming, S.L.; Zhen, S.J.; Lin, K.W.; Zhao, L.; Xu, J.K.; Lu, B.Y. Thiadiazolo[3,4-c]pyridine as an Acceptor toward Fast-Switching Green Donor-Acceptor-Type Electrochromic Polymer with Low Bandgap. ACS Appl. Mater. Interfaces 2015, 7, 11089–11098. [Google Scholar] [CrossRef] [PubMed]
- Henckens, A.; Colladet, K.; Fourier, S.; Cleij, T.J.; Lutsen, L.; Gelan, J.; Vanderzande, D. Synthesis of 3,4-Diphenyl-Substituted Poly(Thienylene Vinylene), Low-Band-Gap Polymers via the Dithiocarbamate Route. Macromolecules 2005, 38, 19–26. [Google Scholar] [CrossRef]
- Thompson, B.C.; Kim, Y.G.; McCarley, T.D.; Reynolds, J.R. Soluble Narrow Band Gap and Blue Propylenedioxythiophene-Cyanovinylene Polymers as Multifunctional Materials for Photovoltaic and Electrochromic Applications. J. Am. Chem. Soc. 2006, 128, 12714–12725. [Google Scholar] [CrossRef] [PubMed]
- Polec, I.; Henckens, A.; Goris, L.; Nicolas, M.; Loi, M.A.; Adriaenses, P.J.; Lutsen, L.; Manca, J.V.; Vanderzande, D.; Sariciftci, N.S. Convenient Synthesis and Polymerization of 5,6-Disubstituted Dithiophthalides Toward Soluble Poly(Isothianaphthene): An Initial Spectroscopic Characterization of the Resulting Low-Band-Gap Polymers. J. Polym. Sci. Part A. 2003, 41, 1034–1045. [Google Scholar] [CrossRef]
- Sheina, E.E.; Khersonsky, S.M.; Jones, E.G.; McCullough, R.D. Highly Conductive, Regioregular Alkoxy-Functionalized Polythiophenes: A New Class of Stable, Low Band Gap Materials. Chem. Mater. 2005, 17, 3317–3319. [Google Scholar] [CrossRef]
- Abdulrazzaq, M.; Ozkut, M.I.; Gokce, G.; Ertan, S.; Tutuncu, E.; Cihaner, A. A Low Band Gap Polymer Based on Selenophene and Benzobis(thiadiazole). Electrochim. Acta 2017, 249, 189–197. [Google Scholar] [CrossRef]
- Havinga, E.E.; Hoeve, W.T.; Wynberg, H. Alternate donor-acceptor small-band-gap semiconducting polymers; Polysquaraines and polycroconaines. Synth. Met. 1993, 55, 299–306. [Google Scholar] [CrossRef]
- Wagner, K.; Pringle, J.M.; Hall, S.B.; Forsyth, M.; MacFarlane, D.R.; Officer, D.L. Investigation of the electropolymerisation of EDOT in ionic liquids. Synth. Met. 2005, 153, 257–260. [Google Scholar] [CrossRef]
- Jamal, R.; Zhang, L.; Wang, M.C.; Zhao, Q.; Abdiryim, T. Synthesis of poly(3,4-propylenedioxythiophene)/MnO2 composites and their applications in the adsorptive removal of methylene blue. Prog. Nat. Sci. Mater. Int. 2016, 26, 32–40. [Google Scholar] [CrossRef]
- Ponder, J.F.; Pittelli, S.L.; Reynolds, J.R. Heteroatom Role in Polymeric Dioxyselenophene/Dioxythiophene Systems for Color and Redox Control. ACS Macro Lett. 2016, 5, 714–717. [Google Scholar] [CrossRef]
- Godeau, G.; Taher, Y.B.; Pujol, M.; Guittard, F.; Darmanin, T. Perfluorinated ProDOT monomers for superhydrophobic/oleophobic surfaces elaboration. J. Fluor. Chem. 2016, 191, 90–96. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Z.G.; Zhang, S.Y.; Li, Y.F. Conjugated Side-Chain-Isolated D–A Copolymers Based on Benzo[1,2-b:4,5-b′]dithiophene-alt-dithienylbenzotriazole: Synthesis and Photovoltaic Properties. Chem. Mater. 2012, 24, 3247–3254. [Google Scholar] [CrossRef]
- Liu, X.C.; Cai, P.; Chen, Z.H.; Zhang, L.J.; Zhang, X.F.; Sun, J.M.; Wang, H.T.; Chen, J.W.; Peng, J.B.; Chen, H.Z.; et al. D-A copolymers based on 5,6-difluorobenzotriazole and oligothiophenes: Synthesis, field effect transistors, and polymer solar cells. Polymer 2014, 55, 1707–1715. [Google Scholar] [CrossRef]
- Li, K.; Li, Z.J.; Feng, K.; Xu, X.P.; Wang, L.Y.; Peng, Q. Development of large band-gap conjugated copolymers for efficient regular single and tandem organic solar cells. J. Am. Chem. Soc. 2013, 135, 13549–13557. [Google Scholar] [CrossRef] [PubMed]
- Genene, Z.; Wang, J.Y.; Meng, X.Y.; Ma, W.; Xu, X.F.; Yang, R.Q.; Mammo, W.; Wang, E.G. High bandgap (1.9 eV) polymer with over 8% efficiency in bulk heterojunction solar cells. Adv. Electron. Mater. 2016, 2, 1600084. [Google Scholar] [CrossRef]
- Todd, A.D.; Bielawski, C.W. Controlled synthesis of an alternating donor-acceptor conjugated polymer via kumada catalyst-transfer polycondensation. ACS Macro Lett. 2015, 4, 1254–1258. [Google Scholar] [CrossRef]
- He, B.; Neo, W.T.; Chen, T.L.; Klivansky, L.M.; Wang, H.X.; Tan, T.W.; Teat, S.J.; Xu, J.W.; Liu, Y. Low bandgap conjugated polymers based on a nature-inspired bay-annulated indigo(BAI) acceptor as stable electrochromic materials. ACS Sustain. Chem. Eng. 2016, 4, 2797–2805. [Google Scholar] [CrossRef]
- Reenes, B.D.; Grenier, C.R.; Argun, A.A.; Cirpan, A.; Mccarley, T.D.; Reynolds, J.R. Spray Coatable Electrochromic Dioxythiophene Polymers with High Coloration Efficiencies. Macromolecules 2004, 37, 7559–7569. [Google Scholar]
- Xie, H.H.; Wang, M.; Kong, L.Q.; Zhang, Y.; Ju, X.P.; Zhao, J.S. The optimization of donor-to-acceptor feed ratios with the aim of obtaining black-to-transmissive switching polymers based on isoindigo as the electron-deficient moiety. RSC Adv. 2017, 7, 11840–11851. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamamoto, S.I.; Koizumi, T. Effects of molecular weight on the optical and electrochemical properties of EDOT-based π-conjugated polymers. Sci. Rep. 2017, 7, 1078. [Google Scholar] [CrossRef] [PubMed]
- Kerszulis, J.A.; Johnson, K.E.; Kuepfert, M.; Khoshabo, D.; Dyer, A.L.; Reynolds, J.R. Tuning the painter’s palette: Subtle steric effects on spectra and colour in conjugated electrochromic polymers. J. Mater. Chem. C 2015, 3, 3211–3218. [Google Scholar] [CrossRef]
- Sun, Q.J.; Wang, H.Q.; Yang, C.H.; Li, Y.F. Synthesis and electroluminescence of novel copolymers containing crown ether spacers. J. Mater. Chem. 2003, 13, 800–806. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Graham, K.R.; Grenier, C.R.; Reynolds, J.R. Influence of the film thickness and morphology on the colorimetric properties of spray-coated electrochromic disubstituted 3,4-propylenedioxythiophene polymers. ACS Appl. Mater. Interfaces 2009, 1, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Navigating the Color Palette of Solution-Processable Electrochromic Polymers. Chem. Mater. 2011, 23, 397–415. [Google Scholar] [CrossRef]
- Zhou, P.; Wan, Z.Q.; Liu, Y.N.; Jia, C.Y.; Weng, X.L.; Xie, J.L.; Deng, L.J. Synthesis and electrochromic properties of a novel conducting polymer film based on dithiafulvenyl-triphenylamine-di(N-carbazole). Electrochim. Acta 2016, 190, 1015–1024. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lu, H.Y. Electrosynthesis of Aromatic Poly(amide-amine) Films from Triphenylamine-Based Electroactive Compounds for Electrochromic Applications. Polymers 2017, 9, 708. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.L.; Ju, X.P.; Zhang, Y.; Zhao, J.S. Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor. Polymers 2017, 9, 173. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Cai, W.A.; Niu, H.J.; Wang, W.; Bai, X.D.; Hou, Y.J. Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties. Polymers 2017, 9, 542. [Google Scholar] [CrossRef]











| Polymer | λonset (film) (nm) | λmax (solution) (nm) | λmax (film) (nm) | Eonset (V) | Eg (eV) | HOMO (eV) | LUMO (eV) |
|---|---|---|---|---|---|---|---|
| P1 | 703 | 504 | 526 | 0.89 | 1.77 | −5.29 | −3.52 |
| P2 | 674 | 502 | 507 | 0.55 | 1.84 | −4.95 | −3.11 |
| P3 | 668 | 502 | 502 | 0.49 | 1.86 | −4.89 | −3.03 |
| Polymer | λ (nm) | ΔT% (%) | Response Time (t95%) (s) | CE Value (cm2·C−1) |
|---|---|---|---|---|
| P1 | 520 1500 | 18.9 32.1 | 2.56 2.44 | 105.54 178.22 |
| P2 | 500 1385 | 40.2 36.4 | 1.31 1.03 | 170.76 184.40 |
| P3 | 505 1400 | 41.4 46.1 | 0.54 2.00 | 110.62 364.95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, F.; Kong, L.; Du, H.; Zhao, J.; Zhang, J. Donor-Acceptor-Type Copolymers Based on 3,4-Propylenedioxy-thiophene and 5,6-Difluorobenzotriazole: Synthesis and Electrochromic Properties. Polymers 2018, 10, 427. https://doi.org/10.3390/polym10040427
Feng F, Kong L, Du H, Zhao J, Zhang J. Donor-Acceptor-Type Copolymers Based on 3,4-Propylenedioxy-thiophene and 5,6-Difluorobenzotriazole: Synthesis and Electrochromic Properties. Polymers. 2018; 10(4):427. https://doi.org/10.3390/polym10040427
Chicago/Turabian StyleFeng, Fanda, Lingqian Kong, Hongmei Du, Jinsheng Zhao, and Junhong Zhang. 2018. "Donor-Acceptor-Type Copolymers Based on 3,4-Propylenedioxy-thiophene and 5,6-Difluorobenzotriazole: Synthesis and Electrochromic Properties" Polymers 10, no. 4: 427. https://doi.org/10.3390/polym10040427
APA StyleFeng, F., Kong, L., Du, H., Zhao, J., & Zhang, J. (2018). Donor-Acceptor-Type Copolymers Based on 3,4-Propylenedioxy-thiophene and 5,6-Difluorobenzotriazole: Synthesis and Electrochromic Properties. Polymers, 10(4), 427. https://doi.org/10.3390/polym10040427