Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy
Abstract
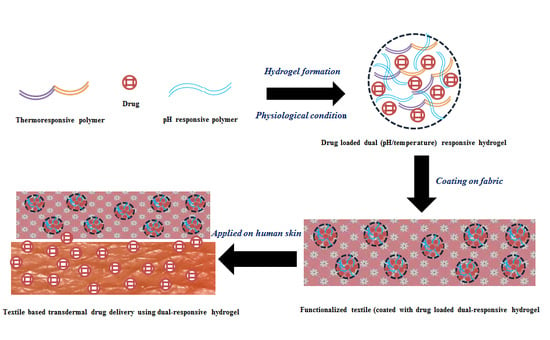
:1. Introduction
2. Natural Polymers
2.1. Chitosan
2.2. Cellulose
2.3. Gelatin/Collagen
3. Synthetic Polymers
3.1. Poly(N-isopropylacrylamide)
3.2. Pluronics or Poloxamers
4. A comparison of the Different Copolymers with Their Benefits and Disadvantages
5. Challenges and Potential New Applications/Commercialization
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels: A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Wood, K.M.; Blanchette, J.O. Hydrogels for oral delivery of therapeutic proteins. Expert Opin. Biol. Ther. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roorda, W.E.; Bodde, H.E.; Boer, A.G.D.; Junginger, H.E. Synthetic hydrogels as drug delivery systems. Pharm. Weekbl. Sci. Ed. 1986, 8, 165–189. [Google Scholar]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 6, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Tomme, S.R.V.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lia, Q.; Liu, C.; Wen, J.; Wu, Y.; Shan, Y.; Liao, J. The design, mechanism and biomedical application of self-healing hydrogels. Chin. Chem. Lett. 2017, 28, 1857–1874. [Google Scholar] [CrossRef]
- Gyarmati, B.; Szilágyi, B.Á.; Szilágyi, A. Reversible interactions in self-healing and shape memory hydrogels. Eur. Polym. J. 2017, 9, 642–679. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications—A review. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Peppas, N.A. Physiologically responsive gels. J. Bioact. Compat. Polym. 1991, 6, 241–246. [Google Scholar] [CrossRef]
- Mastropietro, D.J.; Omidian, H.; Park, K. Drug delivery applications for superporous hydrogels. Expert Opin. Drug. Deliv. 2012, 9, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.L.; Peppas, N.A. Biomedical membranes from hydrogels and interpolymer complexes. Adv. Polym. Sci. 1995, 122, 125–175. [Google Scholar]
- Katchalsky, A.; Michaeli, I. Polyelectrolyte gels in salt solution. J. Polym. Sci. 1955, 15, 69–86. [Google Scholar] [CrossRef]
- Ricka, J.; Tanaka, T. Swelling of ionic gels: Quantitative performance of the Donnan theory. Macromolecules 1984, 17, 2916–2921. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Maolin, Z.; Jun, L.; Min, Y.; Hongfei, H. The swelling behaviour of radiation prepared semi-interpenetrating polymer networks composed of polyNIPAAm and hydrophilic polymers. Radiat. Phys. Chem. 2000, 58, 397–400. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical applications: their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2009, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Kumar, N.; Kumar, M. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Khan, M.; Tamama, K.; Kuppusamy, P.; Wagner, W.R.; Sen, C.K.; Guan, J. Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers. Acta Biomater. 2010, 6, 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Shantha, K.L.; Harding, D.R.K. Synthesis and evaluation of sucrose-containing polymeric hydrogels for oral drug delivery. J. Appl. Polym. Sci. 2002, 84, 2597–2604. [Google Scholar] [CrossRef]
- Wen, Z.; Xing, J.; Yang, C.; Yuying, L.; Jun, F. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Dorkoosh, F.A.; Verhoef, J.C.; Borchard, G.; Rafiee-Tehrani, M.; Verheijden, J.H.M.; Junginger, H.E. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int. J. Pharm. 2002, 247, 47–55. [Google Scholar] [CrossRef]
- Elbert, D.L. Liquid-liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: A tutorial review. Acta Biomater. 2011, 7, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Langer, R. New challenges in biomaterials. Science 1994, 263, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Hydrogels and drug delivery. Curr. Opin. Coll. Int. Sci. 1997, 2, 531–537. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, I.S.; Galante, R.S.C.; Dua, K.; Malipeddi, V.R.; Awasthi, R.; Ghisleni, D.D.M.; Pinto, T.D.J.A. Hydrogel based drug delivery systems: A review with special emphasis on challenges associated with decontamination of hydrogels and biomaterials. Curr. Drug Deliv. 2017, 14, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J. In situ-forming hydrogels–Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Oltarzhevskaya, N.D.; Korovina, M.A. Medical textile and hydrogel materials for targeted delivery of drugs in oncological practice. Russ. J. Gen. Chem. 2012, 82, 2294–2305. [Google Scholar] [CrossRef]
- Bashari, A.; Nejad, N.H.; Pourjavadi, A. Applications of stimuli responsive hydrogels: A textile engineering approach. J. Text. Inst. 2013, 104, 1145–1155. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hui, P.C.L.; Wat, E.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Lau, C.B.S.; Leung, P.-C. Enhanced transdermal permeability via constructing the porous structure of poloxamer-based hydrogel. Polymers 2016, 8, 406. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Yang, Z.; He, L.; Kong, Y.; Fei, B.; Xin, J.H. Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J. The application of temperature-sensitive hydrogels to textiles: A review of Chinese and Japanese investigations. Fibres Text. East Eur. 2005, 13, 45–49. [Google Scholar]
- Wang, J.; Zhong, Q.; Wu, J.; Chen, T. Thermo-responsive textiles. In Handbook of Smart Textiles; Tao, X., Ed.; Springer Science+Business Media: Singapore, 2015; pp. 919–951. ISBN 978-981-4451-46-8. [Google Scholar]
- Vihola, H.; Laukkanen, A.; Tenhu, H.; Hirvonen, J. Drug release characteristics of physically cross-linked thermosensitive poly(N-vinylcaprolactam) hydrogel particles. J. Pharm. Sci. 2008, 97, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Hua, F.; Lee, D.S. Thermoreversible gelation of biodegradable poly(epsilon-caprolactone) and poly (ethylene glycol) multiblock copolymers in aqueous solutions. J. Control. Release 2001, 73, 315–327. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Dong, P.P.; Chen, L.; Wang, X.Z.; Lu, S. Genipin-cross-linked thermosensitive silk sericin/poly(N-isopropylacrylamide) hydrogels for cell proliferation and rapid detachment. J. Biomed. Mater. Res. A 2014, 102, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kozlovskaya, V.; Chen, Y.; Zavgorodnya, O.; Kharlampieva, E. Thermosensitive multilayer hydrogels of poly(N-vinylcaprolactam) as nanothin films and shaped capsules. Chem. Mater. 2012, 24, 3707–3719. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Ding, Q.X.; Fan, M.; Liao, J.F.; Qian, Z.Y.; Luo, J.C.; Li, X.Q.; Luo, F.; Yang, Z.M.; Wei, Y.Q. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials 2014, 35, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Dong, P.W.; Shi, S.; Fu, S.Z.; Yang, J.L.; Guo, G.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. Thermosensitive PEG-PCL-PEG hydrogel controlled drug delivery system: Sol-gel-sol transition and in vitro drug release study. J. Pharm. Sci. 2009, 98, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Kibbey, M.R.; Birnbaum, J.C.; Won, Y.Y.; Gutowska, A. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA. Macromolecules 2000, 33, 8317–8322. [Google Scholar] [CrossRef]
- Lingyun, C.; Zhigang, T.; Yumin, D. Synthesis and pH sensitivity of carboxymethyl chitosan based polyampholyte hydrogel for protein carrier matrices. Biomaterials 2004, 25, 3725–3732. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Sun, H.; Chen, S. Assessment of release kinetics, stability and polymer interaction of poloxamer 407-based thermosensitive gel of interleukin-1 receptor antagonist. Pharm. Dev. Technol. 2014, 19, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Suh, J.M.; Sohn, Y.S.; Bae, Y.H.; Kim, S.W.; Jeong, B. Thermogelling poly(caprolactone-b-ethylene glycol-b-caprolactone) aqueous solutions. Macromolecules 2005, 38, 5260–5265. [Google Scholar] [CrossRef]
- Bromberg, L.E.; Ron, E.S. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug Deliv. Rev. 1998, 31, 197–221. [Google Scholar] [CrossRef]
- Privalov, P.L.; Potekhin, S.A. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986, 131, 4–51. [Google Scholar] [PubMed]
- Hwang, M.J.; Joo, M.K.; Choi, B.G.; Park, M.H.; Hamley, I.W.; Jeong, B. Multiple Sol-Gel transitions of PEG-PCLPEG triblock copolymer aqueous solution. Macromol. Rapid Commun. 2010, 31, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.D.; Cerankowski, L.D. Preparation of films exhibiting balanced temperature dependence to permeation by aqueous solutions-a study of lower consolute behaviour. J. Polym. Sci. Polym. Chem. 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Mah, E.; Ghosh, R. Thermo-responsive hydrogels for stimuli-responsive membranes. Processes 2013, 1, 238–262. [Google Scholar] [CrossRef]
- Southall, N.T.; Dill, K.A.; Haymet, A.D.J. A view of the hydrophobic effect. J. Phys. Chem. B 2002, 106, 521–533. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Poly(ethylene glycol)-poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J. Control. Release 2013, 172, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Hirose, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K. Copolymerization of 2-carboxyisopropylacrylamide with N-isopropylacrylamide accelerates cell detachment from grafted surfaces of reducing temperature. Biomacromolecules 2003, 4, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Denkbas, E.B.; Ottenbrite, R.M. Perspectives on: Chitosan drug delivery systems based on their geometries. J. Bioact. Compat. Polym. 2006, 21, 351–368. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhu, K.J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000, 201, 51–58. [Google Scholar] [CrossRef]
- Dergunov, S.A.; Mun, G.A. γ-Irradiated chitosan-polyvinyl pyrrolidone hydrogels as pH-sensitive protein delivery system. Radiat. Phys. Chem. 2009, 78, 65–68. [Google Scholar] [CrossRef]
- Gong, S.; Tu, H.; Zheng, H.; Xu, H.; Yin, Y. Chitosan-g-PAA hydrogels for colon-specific drug delivery: Preparation, swelling behavior and in vitro degradability. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2010, 25, 248–251. [Google Scholar] [CrossRef]
- Azab, A.K.; Kleinstern, J.; Doviner, V.; Orkin, B.; Srebnik, M.; Nissan, A.; Rubinstein, A. Prevention of tumor recurrence and distant metastasis formation in a breast cancer mouse model by biodegradable implant of 131I-norcholesterol. J. Control. Release 2007, 123, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermoreversible hydrogel. Macromol. Biosci. 2005, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, J.P.; Leu, Y.L.; Hu, J.-W. Temperature-sensitive hydrogels composed of chitosan and hyaluronic acid as injectable carriers for drug delivery. Eur. J. Pharm. Biopharm. 2008, 68, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, S.H.; Park, K.D.; Jung, M.C.; Yang, W.I.; Han, S.W.; Noh, J.Y.; Lee, J.W. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials 2004, 25, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Kennedy, J.F. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohyd. Polym. 2008, 73, 265–273. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Grobner, G. Chitosan in situ gelation for improved drug loading and retention in poloxamer 407 gels. Int. J. Pharm. 2011, 409, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Sun, J.; Zi, P.; Jin, X.; Zhang, C. Thermosensitive micelles-hydrogel hybrid system based on poloxamer 407 for localized delivery of paclitaxel. J. Pharm. Sci. 2013, 102, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hui, P.C.L.; Kan, C.W. Functionalized textile based therapy for the treatment of atopic dermatitis. Coatings 2017, 7, 82. [Google Scholar] [CrossRef]
- Hui, P.C.-L.; Wang, W.-Y.; Kan, C.-W.; Ng, F.S.-F.; Zhou, C.-E.; Wat, E.; Zhang, V.X.; Chan, C.-L.; Lau, C.B.-S.; Leung, P.-C. Preparation and characterization of chitosan/sodium alginate (CSA) microcapsule containing Cortex Moutan. Colloid Surf. A 2013, 434, 95–101. [Google Scholar] [CrossRef]
- Hui, P.C.; Wang, W.Y.; Kan, C.W.; Ng, F.S.; Wat, E.; Zhang, V.X.; Chan, C.L.; Lau, C.B.; Leung, P.C. Microencapsulation of Traditional Chinese Herbs-PentaHerbs extracts and potential application in healthcare textiles. Colloid Surf. B 2013, 111, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, L.; Durai, B.; Rathinamoorthy, R. Manuka honey loaded chitosan hydrogel films for wound dressing applications. Int. J. PharmTech. Res. 2013, 5, 1774–1785. [Google Scholar]
- Risbud, M.V.; Karamuk, E.; Mayer, J. Designing hydrogel coated textile scaffolds for tissue engineering: Effect of casting conditions and degradation behavior studied at microstructure level. J. Mater. Sci. Lett. 2002, 21, 1191–1194. [Google Scholar] [CrossRef]
- Risbud, M.V.; Karamuk, E.; Schlosser, V.; Mayer, J. Hydrogel-coated textile scaffolds as candidate in liver tissue engineering: II. Evaluation of spheroid formation and viability of hepatocytes. J. Biomat. Sci. Polym. Ed. 2003, 14, 719–731. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Maharjan, B.; Liao, N.; Kim, H.J.; Par, C.H.; Kim, C.S. Three-dimensional cellulose sponge: Fabrication, characterization, biomimetic mineralization, and in vitro cell infiltration. Carbohydr. Polym. 2016, 136, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.J.D.; Díaz, A.; Puiggalí, J. Hydrogels for biomedical applications: Cellulose, chitosan, and protein/peptide derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Korpinen, R.; Mikkonen, K.; Willför, S.; Xu, C. Nanofibrillated cellulose originated from birch sawdust after sequential extractions: A promising polymeric material from waste to films. Cellulose 2014, 21, 2587–2598. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels-scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Stella, J.A.; D’Amore, A.; Wagner, W.R.; Sacks, M.S. On the biomechanical function of scaffolds for engineering load-bearing soft tissues. Acta Biomater. 2010, 6, 2365–2381. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Li, L.; Shan, H.; Yue, C.Y.; Lam, Y.C.; Tam, K.C.; Hu, X. Thermally induced association and dissociation of methylcellulose in aqueous solutions. Langmuir 2002, 18, 7291–7298. [Google Scholar] [CrossRef]
- Takahashi, M.; Shimazaki, M.; Yamamoto, J. Thermoreversible gelation and phase separation in aqueous methyl cellulose solutions. J. Polym. Sci. B 2001, 39, 91–100. [Google Scholar] [CrossRef]
- Liang, H.F.; Hong, M.H.; Ho, R.M.; Chung, C.K.; Lin, Y.H.; Chen, C.H.; Sung, H.W. Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Stabenfeldt, S.E.; Garcia, A.J.; LaPlaca, M.C. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, B.; Lu, W.W.; Li, X.; Zhu, D.; Yao, K.D.; Wang, Q.; Zhao, C.; Wang, C. A rapid temperature-responsive sol-gel reversible poly(N-isopropylacrylamide)-g-methylcellulose copolymer hydrogel. Biomaterials 2004, 25, 3005–3012. [Google Scholar] [CrossRef] [PubMed]
- Tomsic, M.; Prossnigg, F.; Glatter, O. A thermoreversible double gel: Characterization of a methylcellulose and kappa-carrageenan mixed system in water by SAXS, DSC and rheology. J. Colloid Interface Sci. 2008, 322, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Wang, X.Y.; Li, Y.; Lei, M.; Du, Y.; Kennedy, J.F.; Knill, C.J. Production and characterisation of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr. Polym. 2010, 82, 833–841. [Google Scholar] [CrossRef]
- Kim, J.K.; Won, Y.W.; Lim, K.S.; Kim, Y.H. Low-molecular-weight methylcellulose-based thermo-reversible gel/pluronic micelle combination system for local and sustained docetaxel delivery. Pharm. Res. 2012, 2, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Babla, H.; Han, T.; Das, D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2016, 23, 668–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose-hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Asghar, A.; Henrickson, R.L. Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems. Adv. Food Res. 1982, 28, 231–372. [Google Scholar] [PubMed]
- Lin, N.; Bruzzese, C.; Dufresne, A. Tempo-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Malinen, M.M.; Kanninen, L.K.; Corlu, A.; Isoniemi, H.M.; Lou, Y.-R.; Yliperttula, M.L.; Urtti, A.O. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 2014, 35, 5110–5121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue derived stromal cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dong, H.; Li, Q.; Wang, G.; Cao, X. High strength, biocompatible hydrogels with designable shapes and special hollow-formed character using chitosan and gelatin. Carbohydr. Polym. 2017, 168, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tabata, Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed. 2010, 21, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Santiago, T.-D.G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Joly-Duhamel, C.; Hellio, D.; Djabourov, M. All gelatin networks: 1. biodiversity and physical chemistry. Langmuir 2002, 18, 7208–7217. [Google Scholar] [CrossRef]
- Chang, Y.; Xiao, L.; Tang, Q. Preparation and characterization of a novel thermosensitive hydrogel based on chitosan and gelatin blends. J. Appl. Polym. Sci. 2009, 113, 400–407. [Google Scholar] [CrossRef]
- Ohya, S.; Matsuda, T. Poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as thermoresponsive three-dimensional artificial extracellular matrix: Molecular and formulation parameters vs. cell proliferation potential. J. Biomater. Sci. Polym. Ed. 2005, 16, 809–827. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Frankowski, D.J.; Spontak, R.J.; Hudson, S.M. Swelling behavior and morphological evolution of mixed gelatin/silk fibroin hydrogels. Biomacromolecules 2005, 6, 3079–3087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Yang, S.-H.; Su, W.-Y.; Chen, Y.C.; Yang, K.C.; Cheng, W.T.; Wu, S.C.; Lin, F.H. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: An in vitro study. Tissue Eng. Pt. A 2010, 16, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kao, W.J. Thermoresponsive gelatin/monomethoxy poly(ethylene glycol)-poly(d,l-lactide) hydrogels: Formulation, characterization, and antibacterial drug delivery. Pharm. Res. 2006, 23, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, Q.; Yuan, S.; Zhang, R.; Wu, Z. Properties of thermoresponsive N-maleyl gelatin-co-P(N-isopropylacrylamide) hydrogel with ultrahigh mechanical strength and self-recovery. J. Polym. Res. 2017, 24, 190. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Liu, Y.; Chen, M.; Hu, Y.; Yang, Z. Study on the grafting of chitosan-gelatin microcapsules onto cotton fabrics and its antibacterial effect. Colloid Surf. B 2013, 109, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Tamayol, A.; Laforte, V.; Annabi, N.; Najafabadi, A.H.; Khademhosseini, A.; Juncker, D. Composite living fibers for creating tissue constructs using textile techniques. Adv. Funct. Mater. 2014, 24, 4060–4067. [Google Scholar] [CrossRef] [PubMed]
- Gulyuz, U.; Okay, O. Self-healing poly(N-isopropylacrylamide) hydrogels. Eur. Polym. J. 2015, 72, 12–22. [Google Scholar] [CrossRef]
- Hacker, M.C.; Klouda, L.; Ma, B.B.; Kretlow, J.D.; Mikos, A.G. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules 2008, 9, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Doorty, K.B.; Golubeva, T.A.; Gorelov, A.V.; Rochev, Y.A.; Allen, L.T.; Dawson, K.A.; Gallagher, W.M.; Keenan, A.K. Poly(N-isopropylacrylamide) co-polymer films as potential vehicles for delivery of an antimitotic agent to vascular smooth muscle cells. Cardiovasc. Pathol. 2003, 12, 105–110. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, J.; Yang, L.; Shi, L.; Tao, Q.; Hui, B.; Li, J. The effect of pH on the LCST of poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylic acid). J. Biomater. Sci. Polym. Ed. 2004, 15, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Yang, Y.-Y.; Chung, T.-S.; Ma, K.-X. Preparation and characterization of fast response macroporous poly(N-isopropylacrylamide) hydrogels. Langmuir 2001, 17, 6094–6099. [Google Scholar] [CrossRef]
- Hirotsu, S. Coexistence of phases and the nature of first-order transition in poly(N-isopropylacrylamide) gels. Adv. Polym. Sci. 1993, 110, 1–26. [Google Scholar]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Coughlan, D.C.; Corrigan, O.I. Drug-polymer interactions and their effect on thermoresponsive poly(N-isopropylacrylamide) drug delivery systems. Int. J. Pharm. 2006, 313, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.; Larson, R.G. Assessing the efficacy of poly(N-isopropylacrylamide) for drug delivery applications using molecular dynamics simulations. Mol. Pharm. 2017, 14, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Soboyejo, W.O. Investigation of swellable poly(N-isopropylacrylamide) based hydrogels for drug delivery. Mat. Sci. Eng. C 2011, 31, 1084–1090. [Google Scholar] [CrossRef]
- Burek, M.; Kowalczyk, M.; Czuba, Z.P.; Krol, W.; Pilawka, R.; Waskiewicz, S. Poly(N-isopropylacrylamide) hydrogels cross-linked by a,a-trehalose diacetals as thermo-responsive and acid-degradable carriers for drug delivery. Polym. Degrad. Stabil. 2016, 129, 296–305. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Ascenzi, P. Poly(N-isopropylacrylamide-coacrylamide) cross-linked thermoresponsive microspheres obtained from preformed polymers: Influence of the physico-chemical characteristics of drugs on their release profiles. Acta Biomater. 2009, 5, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, X.D.; Wang, Z.C.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Synthesis and properties of pH and temperature sensitive P(NIPAAm-co-DMAEMA) hydrogels. Colloid Surf. B 2008, 64, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Trongsatitkul, T.; Budhlall, B.M. Microgels or microcapsules? Role of morphology on the release kinetics of thermoresponsive PNIPAm-co-PEGMa hydrogels. Polym. Chem. 2013, 4, 1502–1516. [Google Scholar] [CrossRef]
- Okuyama, Y.; Yoshida, R.; Sakai, K.; Okano, T.; Sakurai, Y. Swelling controlled zero-order and sigmoidal drug release from thermoresponsive poly(N-isopropylacrylamide-co-butyl methacrylate) hydrogel. J. Biomater. Sci. Polym. Ed. 1993, 4, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Park, J.H.; Kim, S.W.; Sun, B.K.; Woo, D.G.; Chung, H.M.; Park, K.H. Delivery of dexamethasone, ascorbate, and growth factor (TGF β-3) in thermo-reversible hydrogel constructs embedded with rabbit chondrocytes. Biomaterials 2006, 27, 5951–5957. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Gorelov, A.V.; Rochev, Y.A.; McGillicuddy, F.; Dawson, K.A.; Gallagher, W.M.; Keenan, A.K. Extended delivery of the antimitotic agent colchicine from thermoresponsive N-isopropylacrylamide-based copolymer films to human vascular smooth muscle cells. J. Biomed. Mater. Res. A 2003, 67, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.M.; Chellamuthu, P.; Tang, L.; Nguyen, K.T. Development of a temperature-sensitive composite hydrogel for drug delivery applications. Biotechnol. Prog. 2006, 22, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Derwent, J.J.K.; Mieler, W.F. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206–214. [Google Scholar]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T.; Miyazaki, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control. Release 2006, 115, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, H.; Hendrix, M.M.; Lousberg, N.J.; With, G.D.; Esteves, A.C.C.; Xin, J.H. Temperature-triggered collection and release of water from fogs by a sponge-like cotton fabric. Adv. Mater. 2013, 25, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Schiphorst, J.T.; Broek, M.V.D.; Koning, T.D.; Murphy, J.N.; Schenning, A.P.H.J.; Esteves, A.C.C. Dual light and temperature responsive cotton fabric functionalized with a surface-grafted spiropyran-NIPAAm-hydrogel. J. Mater. Chem. A 2016, 4, 8676–8681. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Q.; Wang, T. Thermoresponsive PNIPAAm-modified cotton fabric surfaces that switch between superhydrophilicity and superhydrophobicity. Appl. Surf. Sci. 2012, 258, 4888–4892. [Google Scholar] [CrossRef]
- Bashari, A.; Hemmatinejad, N.; Pourjavadi, A. Surface modification of cotton fabric with dual-responsive PNIPAAm/chitosan nano hydrogel. Polym. Adv. Technol. 2013, 24, 797–806. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J.; Meng, Q. Nonwoven supported temperature-sensitive poly(N-isopropylacrylamide)/polyurethane copolymer hydrogel with antibacterial activity. J. Biomed. Mater. Res. B 2009, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and interfaces: Thermodynamics, structure, dynamics and modeling. Colloid Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, M.; Fang, Q.; Zheng, H.; Wu, F.; Lin, D.; Xu, Y.; Jin, Y. Preparation and in vitro characterization of pluronic-attached polyamidoamine dendrimers for drug delivery. Drug Dev. Ind. Pharm. 2015, 41, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.; Zhu, J.; Alakhov, V. Pluronic Block Copolymers for Gene Delivery. Adv. Genet. 2005, 53, 231–261. [Google Scholar] [PubMed]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Yap, L.S.; Yang, M.C. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloid Surf. B 2016, 146, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Li, N.; Gao, J.-Q.; Sun, H.; Chen, S. Sustained delivery of IL1Ra from Pluronic F127-based thermosensitive gel prolongs its therapeutic potentials. Pharm. Res. 2012, 29, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Aoki, N.; Yamamoto, T.; Gomei, Y.; Egashira, S.; Matsuoka, Y.; Miyazaki, T.; Fukushima, H.; Lee, Y.M.; Jyujyoji, S.; et al. Bio-inert surface of Pluronic-immobilized flask for preservation of haematopoietic stem cells. Biomacromolecules 2006, 7, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Sugiyama, K.; Yoon, B.O.; Sakurai, M.; Hara, M.; Sumita, M.; Sugawara, S.; Shirai, T. Serum protein adsorption and platelet adhesion on pluronic-adsorbed polysulfone membranes. Biomaterials 2003, 24, 3235–3245. [Google Scholar] [CrossRef]
- Matthew, J.E.; Nazario, Y.L.; Roberts, S.C.; Bhatia, S.R. Effect of mammalian cell culture medium on the gelation properties of pluronic F127. Biomaterials 2002, 23, 4615–4619. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Chen, Y.; Ye, J.; Sha, X.; Fang, X. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Zhang, X.; Lu, W.; Zhang, Q. A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J. Control. Release 2009, 135, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Boca, S.C.; Astilean, S. Pluronic-nanogold hybrids: Synthesis and tagging with photosensitizing molecules. Colloid Surf. B 2012, 97, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hsu, S.H.; Leu, Y.L.; Hu, J.W. Delivery of cisplatin from pluronic co-polymer systems: Liposome inclusion and alginate coupling. J. Biomater. Sci. Polym. Ed. 2009, 20, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Wu, H.-C.; Sun, J.-S.; Dong, G.-C.; Wang, T.-W. Injectable and thermoresponsive self-assembled nanocomposite hydrogel for long-term anticancer drug delivery. Langmuir 2013, 29, 3721–3729. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug 2002, 19, 1–72. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, L.; Dong, H.Q.; Cai, X.J.; Ren, T.B. Pluronic F127 nanomicelles engineered with nuclear localized functionality for targeted drug delivery. Mat. Sci. Eng. C 2013, 33, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.D.; Xu, C.X.; Quan, J.S.; Song, C.K.; Jin, H.; Kim, D.D.; Choi, Y.J.; Cho, M.H.; Cho, C.S. Synergistic anti-tumor activity of paclitaxel-incorporated conjugated linoleic acid coupled poloxamer thermosensitive hydrogel in vitro and in vivo. Biomaterials 2009, 30, 4777–4785. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhu, L.; Xia, S.; Zhang, X. Dual pH-/temperature-responsive and fluorescent hydrogel for controlled drug delivery. J. Polym. Eng. 2018, 38, 371–379. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, P. Versatile injectable supramolecular hydrogels containing drug loaded micelles for delivery of various drugs. Polym. Chem. 2014, 5, 1072–1081. [Google Scholar] [CrossRef]
- Rokhade, A.P.; Shelke, N.B.; Patil, S.A.; Aminabhavi, T.M. Novel hydrogel microspheres of chitosan and pluronic F-127 for controlled release of 5-fluorouracil. J. Microencapsul. 2007, 24, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Leu, Y.-L.; Hu, J.-W.; Fang, J.-Y. Physicochemical characterization and drug release of thermosensitive hydrogels composed of a hyaluronic acid/pluronic F127 graft. Chem. Pharm. Bull. 2009, 57, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, X.; Ma, J.; Hao, D.; Wei, P.; Zhou, L.; Liu, G. Evaluation of TPGS-modified thermo-sensitive Pluronic PF127 hydrogel as a potential carrier to reverse theresistance of P-gp-overexpressing SMMC-7721 cell lines. Colloid Surf. B 2016, 140, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Khateb, K.A.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on PluronicF127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yapar, E.A.; Ýnal, Ö. Poly(ethylene oxide)-poly (propylene oxide)-based copolymers fortransdermal drug delivery: An overview. Trop. J. Pharm. Res. 2013, 11, 855–866. [Google Scholar]
- Wang, W.; Hui, P.C.L.; Wat, E.; Ng, F.S.F.; Kan, C.W.; Wang, X.; Wong, E.C.W.; Hu, H.; Chan, B.; Lau, C.B.S.; et al. In vitro drug release and percutaneous behavior of poloxamer-based hydrogel formulation containing traditional Chinese medicine. Colloid Surf. B 2016, 148, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naik, A.; Kalia, Y.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Pulsatile local delivery of thrombolytic and antithrombotic agents using poly(Nisopropylacrylamide- co-methacrylic acid) hydrogels. J. Control. Release 1996, 39, 57–64. [Google Scholar] [CrossRef]
- Bilia, A.; Carelli, V.; Colo, G.D.; Nannipieri, E. In vitro evaluation of a pH-sensitive hydrogel for control of GI drug delivery from silicone-based matrices. Int. J. Pharm. 1996, 130, 83–92. [Google Scholar] [CrossRef]
- Ito, A.; Dobashi, Y.; Obata, K.; Sugihara, M. Investigation of compressed coating tablet swelling with water as a new dosage form for elderly patients. Jpn. J. Hosp. Pharm. 1994, 20, 41–49. [Google Scholar] [CrossRef]
- Patel, V.R.; Amiji, M.M. Preparation and characterization of freeze-dried chitosan-poly(ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm. Res. 1996, 13, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Amiji, M.; Tailor, R.; Ly, M.-K.; Goreham, J. Gelatin-poly(ethylene oxide) semi-interpenetrating polymer network with pH-sensitive swelling and enzyme-degradable properties for oral drug delivery. Drug Dev. Ind. Pharm. 1997, 23, 575–582. [Google Scholar] [CrossRef]
- Sen, M.; Uzun, C.; Güven, O. Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide/maleic acid) hydrogels. Int. J. Pharm. 2000, 203, 149–157. [Google Scholar] [CrossRef]
- Ferreira, L.; Vidal, M.M.; Gil, M.H. Evaluation of poly(2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int. J. Pharm. 2000, 194, 169–180. [Google Scholar] [CrossRef]
- Serres, A.; Baudys, M.; Kim, S.W. Temperature and pH sensitive polymers for human calcitonin delivery. Pharm. Res. 1996, 13, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.-L.; Zhang, P.-F.; Ren, X.-B.; Zhong, H.-B.; Gou, Y.-B.; Dong, L.-R.; Li, Q. Synthesis and characterization of a pH/temperature responsive glycine-mediated hydrogel for drug release. Front. Mater. Sci. China 2009, 3, 374. [Google Scholar] [CrossRef]



| Natural Polymers | Structure | Process Condition | Properties and Temperature Responsiveness | Biomedical Applications (Drug Delivery System) | Textile Based Applications for Transdermal Therapy |
|---|---|---|---|---|---|
| Chitosan | d-glucosamine units and trace amount of N-acetyl-d-glucosamine | Exoskeleton of crustacean, from chitin by alkaline deacetylation | Carbohydrate biopolymer, biodegradable, biocompatible and effectively showing thermoresponsive properties after modification/conjugation in the form of hydrogels with gel formation at body temperature [15] | Thermosensitive hydrogels using chitosan, hyaluronic acid and N-isopropylacrylamide (NIPAAm) for analgesic drug nalbuphine [85] | Textile based transdermal therapy was done using microcapsules of chitosan and alginate loaded with traditional Chinese medicines (PentaHerbs formula and cortex moutan) [93,94] |
| Cellulose | d-glucopyranose units | Primary cell wall of green plants and many varieties of algae | Natural polysaccharide and various cellulose derivatives like methyl cellulose (MC) forming thermoresponsive hydrogels at 60–80 °C | Thermoresponsive hydrogel system made from MC-pluronic micelle for anticancer drug docetaxel [114] | Textile based transdermal drug delivery system was developed from thermoresponsive hydrogel of PF127 and carboxymethyl cellulose sodium loaded with the Chinese herbal medicine (cortex moutan) [51,52] |
| Gelatin | Amino acids (rich in hydroxyproline proline, glycine) | Animal tissues such as beef bones, cartilage, tendons and pork skin by boiling | Natural polymer made of amino acids and hydrogels made of gelatin derivative show thermoresponsive properties with sol-gel transition at body temperature | Thermoresponsive hydrogel made of gelatin and monomethoxy poly(ethylene glycol)-poly(d,l-lactide) (MPEG-PDLLA) for antibacterial drug gentamicin sulfate [131] | Hydrogel from carboxymethyl cellulose/gelatin copolymer loaded with lidocaine was applied as drug delivery system for transdermal drug delivery [115] |
| Synthetic Polymers | Structural units | Process condition | Properties and temperature responsiveness | Biomedical applications (Drug delivery system) | Textile based applications for transdermal therapy |
| pNIPAAM | N-isopropylacrylamide units | Synthesized from commercially available N-isopropylacrylamide via free-radical polymerization [193] | Synthetic polymer with intrinsic thermoresponsive properties with sol-gel transition at body temperature. Derivatives of polymer are capable of forming thermoresponsive hydrogels with better mechanical properties | Thermoresponsive poly(N-isopropylacrylamide-co-butyl methacrylate) hydrogel for drug indomethacin [150] | - |
| PF127 | Triblock copolymer, central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol | Synthetized by condensation of ethylene oxide and propylene oxide [194] | Amphiphilic synthetic polymer with intrinsic thermoresponsive properties and can form hydrogels in situ, effectively used as injectable polymer for drug delivery applications | Thermoresponsive hydrogel made of polyurethane-PF127- Erythrosine B for anticancer drug doxorubicin [185] | Thermoresponsive hydrogel from PF127 and carboxymethyl cellulose sodium loaded with the Chinese herbal medicine (cortex moutan) was applied for textile based transdermal drug delivery system [51,52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Hui, P.C.-l.; Kan, C.-w. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. https://doi.org/10.3390/polym10050480
Chatterjee S, Hui PC-l, Kan C-w. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers. 2018; 10(5):480. https://doi.org/10.3390/polym10050480
Chicago/Turabian StyleChatterjee, Sudipta, Patrick Chi-leung Hui, and Chi-wai Kan. 2018. "Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy" Polymers 10, no. 5: 480. https://doi.org/10.3390/polym10050480
APA StyleChatterjee, S., Hui, P. C. -l., & Kan, C. -w. (2018). Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers, 10(5), 480. https://doi.org/10.3390/polym10050480