PP/TiO2 Melt-Blown Membranes for Oil/Water Separation and Photocatalysis: Manufacturing Techniques and Property Evaluations
Abstract
:1. Introduction
2. Experimental
2.1. Materials
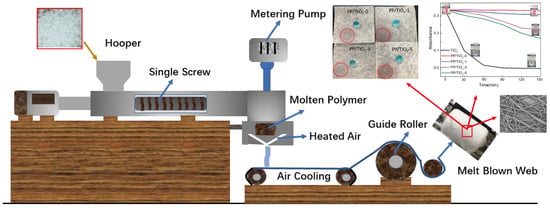
2.2. Preparation of Melt-Blown Membranes
2.3. Measurements and Characterizations
2.4. Oil/Water Separation and Photocatalytic Activity Analysis
3. Results and Discussion
3.1. Thermal Behaviors and Thermal Stability of the PP/TiO2 Master Batch
3.2. Morphology of PP/TiO2 Hot-Press Film and Melt-Blown Membranes
3.3. XRD and FTIR Analysis of PP/TiO2 Melt-Blown Membrances
3.4. Oil/Water Separation of PP/TiO2 Melt-Blown Membranes
3.5. Photocatalysis of PP/TiO2 Melt-Blown Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.; Xu, Z. Mineral-Coated Polymer Membranes with Superhydrophilicity and Underwater Superoleophobicity for Effective Oil/Water Separation. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Ali, N.; Zhang, B.; Zhang, H.; Zaman, W.; Li, X.; Li, W.; Zhang, Q. Interfacially active and magnetically responsive composite nanoparticles with raspberry like structure; synthesis and its applications for heavy crude oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 472, 38–49. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, W.; Zhang, F.; Liu, X.; Wang, D.; Jin, J.; Jian, L. Ultrafast Separation of Emulsified Oil/Water Mixtures by Ultrathin Free-Standing Single-Walled Carbon Nanotube Network Films. Adv. Mater. 2013, 25, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Ventikos, N. A high-level synthesis of oil spill response equipment and countermeasures. J. Hazard. Mater. 2004, 107, 51–58. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically Driven Floating Foams for the Removal of Oil Contaminants from Water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef]
- Dong, X.; Chen, J.; Ma, Y.; Wang, J.; Chan-Park, M.B.; Liu, X.; Wang, L.; Huang, W.; Chen, P. Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem. Commun. 2012, 48, 166–1662. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, P. Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: Toward controllable oil/water separation. NPG Asia Mater. 2012, 4, e8. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, J.; Luo, Z. PhotoATRP-Based Fluorinated Thermosensitive Block Copolymer for Controllable Water/Oil Separation. Ind. Eng. Chem. Res. 2015, 54, 10714–10722. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, N.; Fu, C.; Li, K.; Tao, L.; Feng, L.; Wei, Y. Thermo and pH Dual-Responsive Materials for Controllable Oil/Water Separation. ACS Appl. Mater Interfaces 2014, 6, 2026–2030. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z. pH-responsive bidirectional oil–water separation material. Chem. Commun. 2013, 49, 9416–9418. [Google Scholar] [CrossRef]
- Teng, C.; Lu, X.; Ren, G.; Zhu, Y.; Wan, M.; Jiang, L. Underwater Self-Cleaning PEDOT-PSS Hydrogel Mesh for Effective Separation of Corrosive and Hot Oil/Water Mixtures. Adv. Mater. Interfaces 2014, 1, 1400099. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, D.; Yu, C.; Jiang, L. Facile Fabrication of a Polyethylene Mesh for Oil/Water Separation in a Complex Environment. ACS Appl. Mater. Interfaces 2016, 8, 24186–24191. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Jiang, Z.; Luo, Z. Electrospun Fibrous Mat with pH-Switchable Superwettability That Can Separate Layered Oil/Water Mixtures. Langmuir 2016, 32, 13358–13366. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Liu, L.; Li, T.; Dang, Z.; Qiao, C.; Xu, J.; Wang, Y. Electrospun N-Substituted Polyurethane Membranes with Self-Healing Ability for Self-Cleaning and Oil/Water Separation. Chem. Eur. J. 2016, 22, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Murakami, T.; Fujishima, A. Sol–gel SiO2/TiO2 bilayer films with self-cleaning and antireflection properties. Sol. Energy Mater. Sol. Cells 2008, 92, 1434–1438. [Google Scholar] [CrossRef]
- Pan, J.H.; Wang, X.Z.; Huang, Q.; Shen, C.; Koh, Z.Y.; Wang, Q.; Engel, A.; Bahnemann, D.W. Large-scale Synthesis of Urchin-like Mesoporous TiO2 Hollow Spheres by Targeted Etching and Their Photoelectrochemical Properties. Adv. Funct. Mater. 2014, 24, 95–104. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, C.; Wu, F.; Zou, B.; Zhao, M.; Wang, J.; Feng, C. Photodegradation of rhodamine B under visible light by bimetal codoped TiO2 nanocrystals. J. Hazard. Mater. 2009, 164, 615–620. [Google Scholar] [CrossRef]
- Jo, S.; Kim, Y. Superhydrophilic–underwater superoleophobic TiO2-coated mesh for separation of oil from oily seawater/wastewater. Korean J. Chem. Eng. 2016, 33, 3203–3206. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Hu, W.; Li, D.; Zha, F.; Lei, Z. Facile fabrication of underwater superoleophobic TiO2 coated mesh for highly efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 441–446. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462–463, 178–195. [Google Scholar] [CrossRef]
- Chen, T.; Huang, X. Air drawing of polymers in the melt blowing nonwoven process: Mathematical modelling. Model. Simul. Mater. Sci. Eng. 2004, 12, 381–388. [Google Scholar] [CrossRef]
- Chen, T.; Huang, X. Modeling Polymer Air Drawing in the Melt Blowing Nonwoven Process. Text. Res. J. 2003, 73, 651–654. [Google Scholar] [CrossRef]
- Feng, J. Preparation and properties of poly(lactic acid) fiber melt blown non-woven disordered mats. Mater. Lett. 2017, 189, 180–183. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Song, C.; Gu, Q.; Sang, Y.; Lu, G.; Hu, H.; Li, F. Adsorption-filtration characteristics of melt-blown polypropylene fiber in purification of reclaimed water. Desalination 2006, 201, 198–206. [Google Scholar] [CrossRef]
- Hegde, R.R.; Bhat, G.S. Nanoparticle effects on structure and properties of polypropylene meltblown webs. J. Appl. Polym. Sci. 2010, 115, 1062–1072. [Google Scholar] [CrossRef]
- Song, X.; Zhou, S.; Wang, Y.; Kang, W.; Cheng, B. Mechanical properties and crystallization behavior of polypropylene non-woven fabrics reinforced with POSS and SiO2 nanoparticles. Fiber Polym. 2012, 13, 1015–1022. [Google Scholar] [CrossRef]
- Yu, B.; Han, J.; He, X.; Xu, G.; Ding, X. Effects of tourmaline particles on structure and properties of polypropylene filtration melt-blown nonwoven electrets. J. Macromol. Sci. B 2012, 51, 619–629. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, X.; She, H.; Yang, Y.; Zha, F. Superhydrophobic meshes that can repel hot water and strong corrosive liquids used for efficient gravity-driven oil/water separation. Nanoscale 2016, 8, 1–29. [Google Scholar]
- Jiang, Q.; Pei, X.; Wu, L.; Li, T.T.; Lin, J.H. UV resistance and water barrier properties of PP/PLA/MAH/TiO2 functional hybrid biocomposite films for packaging application. Adv. Polym. Technol. 2018, 37, 2971–2980. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Yu, B.; Wang, M.; Sun, H.; Zhu, F.; Han, J.; Bhat, G. Preparation and properties of poly (lactic acid)/magnetic Fe3O4 composites and nonwovens. RSC Adv. 2017, 7, 41929–41935. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Ding, B.; Zainab, G.; El-Newehy, M.; Al-Deyab, S.S.; Yu, J. In situ cross-linked superwetting nanofibrous membranes for ultrafast oil–water separation. J. Mater. Chem. A. 2014, 2, 10137–10145. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Chen, Z.; Dai, H. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]








| Parameter | Screw 1 Temperature (°C) | Screw 2 Temperature (°C) | Screw 3 Temperature (°C) | Nozzle Temperature (°C) | Screw Speed (r/min) | Pelletized Speed (r/min) |
|---|---|---|---|---|---|---|
| 180 | 200 | 190 | 170 | 21 | 32 |
| Sample | Tmc (°C) | Tg (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| PP/TiO2-0 | 113.60 | 41.50 | 164.90 | 36.29 |
| PP/TiO2-1 | 115.00 | 42.40 | 162.50 | 37.68 |
| PP/TiO2-3 | 115.80 | 42.20 | 163.30 | 42.69 |
| PP/TiO2-5 | 116.00 | 42.10 | 161.90 | 40.81 |
| Sample Type | T0.05 (°C) | T0.5 (°C) | Tmax (°C) | Remnant Mass (%) |
|---|---|---|---|---|
| PP/TiO2-0 | 349.80 | 418.13 | 438.60 | 0.02 |
| PP/TiO2-1 | 350.70 | 421.30 | 440.40 | 0.84 |
| PP/TiO2-3 | 385.50 | 450.40 | 462.60 | 2.95 |
| PP/TiO2-5 | 399.30 | 456.60 | 466.60 | 5.11 |
| Sample | N Total | Mean | Standard Deviation | Sum (μm) | Minimum (μm) | Median (μm) | Maximum (μm) |
|---|---|---|---|---|---|---|---|
| M-PP/TiO2-0 | 88 | 1.99 | 0.54 | 175.37 | 0.94 | 1.86 | 3.56 |
| M-PP/TiO2-1 | 104 | 4.93 | 1.93 | 512.80 | 2.46 | 4.43 | 9.72 |
| M-PP/TiO2-3 | 99 | 5.34 | 2.26 | 529.08 | 1.74 | 5.22 | 9.91 |
| M-PP/TiO2-5 | 93 | 5.78 | 1.95 | 537.76 | 1.74 | 6.09 | 9.84 |
| Acetone:Water (v/v) | M-PP/TiO2-0 | M-PP/TiO2-1 | M-PP/TiO2-3 | M-PP/TiO2-5 |
|---|---|---|---|---|
| 0:5 | 140 | 138 | 134 | 132 |
| 1:4 | 116 | 130 | 132 | 132 |
| 2:3 | 109 | 116 | 120 | 124 |
| 3:2 | 20 | 59 | 76 | 98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Li, T.-T.; Ren, H.; Jiang, Q.; Peng, H.-K.; Lin, Q.; Lou, C.-W.; Lin, J.-H. PP/TiO2 Melt-Blown Membranes for Oil/Water Separation and Photocatalysis: Manufacturing Techniques and Property Evaluations. Polymers 2019, 11, 775. https://doi.org/10.3390/polym11050775
Sun F, Li T-T, Ren H, Jiang Q, Peng H-K, Lin Q, Lou C-W, Lin J-H. PP/TiO2 Melt-Blown Membranes for Oil/Water Separation and Photocatalysis: Manufacturing Techniques and Property Evaluations. Polymers. 2019; 11(5):775. https://doi.org/10.3390/polym11050775
Chicago/Turabian StyleSun, Fei, Ting-Ting Li, Haitao Ren, Qian Jiang, Hao-Kai Peng, Qi Lin, Ching-Wen Lou, and Jia-Horng Lin. 2019. "PP/TiO2 Melt-Blown Membranes for Oil/Water Separation and Photocatalysis: Manufacturing Techniques and Property Evaluations" Polymers 11, no. 5: 775. https://doi.org/10.3390/polym11050775
APA StyleSun, F., Li, T. -T., Ren, H., Jiang, Q., Peng, H. -K., Lin, Q., Lou, C. -W., & Lin, J. -H. (2019). PP/TiO2 Melt-Blown Membranes for Oil/Water Separation and Photocatalysis: Manufacturing Techniques and Property Evaluations. Polymers, 11(5), 775. https://doi.org/10.3390/polym11050775