Reducing off-Flavour in Commercially Available Polyhydroxyalkanoate Materials by Autooxidation through Compounding with Organoclays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
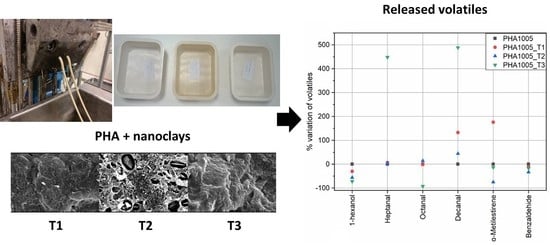
2.2. Nano-Bio-Composites Preparation
2.3. General Characterisation Methods
2.3.1. Scanning Electron Microscopy
2.3.2. Thermogravimetric Analysis
2.3.3. Analysis of Volatile Compounds by HS-SPME-GC-MS
HS-SPME Extraction Procedure
GC-MS Parameters
Optimization of HS-SPME Extraction Parameters Box-Benhken Experimental Design (BBD)
2.3.4. Statistical Analysis
3. Results & Discussion
3.1. SEM—Scanning Electron Microscopy
3.2. TGA—Thermogravimetric Analysis
3.3. HS-SPME-GC-MS—Headspace Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry
3.3.1. Optimization of the HS-SPME Extraction Process
3.3.2. Validation method
Volatile Compounds Quantification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Research and Markets. Global Polyhydroxyalkanoate (PHA) Market Analysis & Trends—Industry Forecast to 2025. Available online: https://www.researchandmarkets.com/reports/4375504/global-polyhydroxyalkanoate-pha-market-analysis (accessed on 1 August 2017).
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Patra, J.K. Polyhydroxyalkanoates: Biodegradable Plastics for Environmental Conservation. In Industrial & Environmental Biotechnology; Studium Press: New Delhi, India, 2014; Chapter 1. [Google Scholar]
- Rehm, B.H.A. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 376, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Iwata, T. Sustainability of Biobased and Biodegradable Plastics. Clean 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Krzyzanek, V.; Mravec, F.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Marova, I. Accumulation of Poly(3-hydroxybutyrate) Helps Bacterial Cells to Survive Freezing. PLoS ONE 2016, 11, e0157778. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Braunegg, G. Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and economic fashion. EuroBiotech J. 2018, 2, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Kabasci, S. Bio-Based Plastics: Materials and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; Chapter 7.3.2; ISBN 9781119994008. [Google Scholar]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Kho, H.-P.; Riedel, S.L.; Kim, S.-W.; Gan, C.-Y.; Taylor, T.D.; Sudesh, K. An integrative study on biologically recovered polyhydroxyalkanoates (PHAs) and simultaneous assessment of gut microbiome in yellow mealworm. J. Biotechnol. 2018, 265, 31–39. [Google Scholar] [CrossRef]
- Brigham, C.J.; Riedel, S.L. The Potential of Polyhydroxyalkanoate Production from Food Wastes. Appl. Food Biotechnol. 2018, 6, 7–18. [Google Scholar] [CrossRef]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.-W.; Wei, Y.-H.; Wu, H.-S.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Fung, F.M.; Su, M.; Feng, H.; Li, S.F.Y. Extraction, separation and characterization of endotoxins in water samples using solid phase extraction and capillary electrophoresis-laser induced fluorescence. Sci. Rep. 2017, 7, 10774. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.J. Oxidative rancidity as a source of off-flavours. In Taints and Off-Flavours in Food; Woodhead Publishing Limited: Cambridge, UK, 2003; pp. 140–158. [Google Scholar]
- Koller, M.; Bona, R.; Chiellini, E.; Braunegg, G. Extraction of short-chain-length poly-[(R)-hydroxyalkanoates] (scl-PHA) by the antisolvent acetone under elevated temperature and pressure. Biotechnol. Lett. 2013, 35, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Darani, K.; Vasheghani-Farahani, E. Application of supercritical fluid extraction in biotechnology. Crit. Rev. Biotechnol. 2005, 25, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kokosa, J.M. Recent Trends in Using Single-Drop Microextraction and Related Techniques in Green Analytical Methods. Trends Anal. Chem. 2015, 71, 194–204. [Google Scholar] [CrossRef]
- Barros, P.; Moreira, E.; Elias Pereira, N.; Leite, G.; Moraes Rezende SG, F.; Guedes de Pinho, P. Development and validation of automatic HS-SPME with a gas chromatography-ion trap/mass spectrometry method for analysis of volatiles in wines. Talanta 2012, 101, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.; Strlič, M. Polymers and volatiles: Using VOC analysis for the conservation of plastic and rubber objects. Stud. Conserv. 2014, 60, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vilaplana, F.; Martínez-Sanz, M.; Ribes-Greus, A.; Karlsson, S. Emission pattern of semi-volatile organic compounds from recycled styrenic polymers using headspace solid-phase microextraction gas chromatography–mass spectrometry. J. Chromatogr. A 2010, 1217, 359–367. [Google Scholar] [CrossRef]
- Kaykhaii, M.; Linford, M.R. Application of Microextraction Techniques Including SPME and MESI to the Thermal Degradation of Polymers: A Review. Crit. Rev. Anal. Chem. 2016, 47, 172–186. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Kaykhaii, M.; Khajeh, M. Molecularly Imprinted Polymers for Stir Bar Sorptive Extraction: Synthesis, Characterization, and Application. Anal. Lett. 2015, 48, 1815–1829. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Franchini, E. Structuration of Nano-Objects in Epoxy-based Polymer Systems: Nanoparticles & Nanoclusters for Improved Fire Retardant Properties. Ph.D. Thesis, Institut National des Sciences Appliquées de Lyon, Lyon, France, 2008. [Google Scholar]
- Volle, N.; Giulieri, F.; Burr, A.; Pagnotta, S.; Chaze, A.M. Controlled interactions between silanol groups at the surface of sepiolite and an acrylate matrix: Consequences on the thermal and mechanical properties. Mater. Chem. Phys. 2012, 134, 417–424. [Google Scholar] [CrossRef]
- Peinado, V.; García, L.; Fernández, A.; Castell, P. Novel lightweight foamed poly(lactic acid) reinforced with different loadings of functionalised Sepiolite. Compos. Sci. Technol. 2014, 101, 17–23. [Google Scholar] [CrossRef]
- Zheng, Y.; Zaoui, A. Mechanical behavior in hydrated Na-montmorillonite clay. Physica A 2018, 505, 582–590. [Google Scholar] [CrossRef]
- Wang, S.; Song, C.; Chen, G.; Guo, T.; Liu, J.; Zhang, B.; Takeuchi, S. Characteristics and biodegradation properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/organophilic montmorillonite (PHBV/OMMT) nanocomposite. Polym. Degrad. Stab. 2005, 87, 69–76. [Google Scholar] [CrossRef]
- García-Quiles, L.; Fernández, A.; Castell, P. Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers 2019, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Félix, J.S.; Domeño, C.; Nerín, C. Characterization of wood plastic composites made from landfill-derived plastic and sawdust: Volatile compounds and olfactometric analysis. Waste Manag. 2013, 33, 645–655. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Egasse, C.; Thao-Heu, S.; Balcar, N.; Barabant, G.; Lavédrine, B. What do plastics emit? HS-SPME-GC/MS analyses of new standard plastics and plastic objects in museum collections. J. Cult. Herit. 2013, 14, 238–247. [Google Scholar] [CrossRef]
- Espert, A.; de las Heras, L.A.; Karlsson, S. Emission of possible odourous low molecular weight compounds in recycled biofibre/polypropylene composites monitored by head-space SPME-GC–MS. Polym. Degrad. Stab. 2005, 90, 555–562. [Google Scholar] [CrossRef]
- Mahesh, K.R.V.; Murthy, H.N.N.; Kumaraswamy, B.E.; Raghavendra, N.; Sridhar, R.; Krishna, M.; Pattar, N.; Pal, R.; Sherigara, B.S. Synthesis and characterization of organomodified Na-MMT using cation and anion surfactants. Front. Chem. China 2011, 6, 153–158. [Google Scholar] [CrossRef]
- Jalali, A.M.; Taromi, F.A.; Atai, M.; Solhi, L. Effect of reaction conditions on silanisation of sepiolite nanoparticles. J. Exp. Nanosci. 2016, 11, 1171–1183. [Google Scholar] [CrossRef] [Green Version]
- González-Ausejo, J.; Gámez-Pérez, J.; Balart, R.; Lagarón, J.M.; Cabedo, L. Effect of the addition of sepiolite on the morphology and properties of melt compounded PHBV/PLA blends. Polym. Compos. 2017. [CrossRef]
- Wang, H.H.; Zhou, X.R.; Liu, Q.; Chen, G.Q. Biosynthesis of polyhydroxyalkanoates homopolymers by Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 89, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Liu, M.; Jia, D. Thermal stability of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/modified montmorillonite bio-nanocomposites. Polym. Compos. 2015, 38, 673–681. [Google Scholar] [CrossRef]
- Tang, D.; Noordover BA, J.; Sablong, R.J.; Koning, C.E. Metal-free synthesis of novel biobased dihydroxyl-terminated aliphatic polyesters as building blocks for thermoplastic polyurethanes. J. Polym. Sci. A Polym. Chem. 2011, 49, 2959–2968. [Google Scholar] [CrossRef]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y. Morphology and functional properties of commercial Polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Ipsita, R.; Visakh, P.M. Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Wypych, G. Handbook of Nucleating Agents; Chemtech Publishing: Toronto, ON, Canada, 2016; ISBN 978-1-895198-93-5. [Google Scholar]
- Tartaglione, G.; Tabuani, D.; Camino, G. Thermal and morphological characterisation of organically modified sepiolite. Microporous Mesoporous Mater. 2008, 107, 161–168. [Google Scholar] [CrossRef]
- Lvov, Y.; Guo, B.; Fakhrullin, R.F. Functional Polymer Composites with Nanoclays; RSC Smart Materials: Cambridge, UK, 2016; ISBN 1782624228. [Google Scholar]
- Lemić, J.; Tomašević-Čanović, M.; Djuričić, M.; Stanić, T. Surface modification of sepiolite with quaternary amines. J. Colloid Interface Sci. 2005, 292, 11–19. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Torres Sanchez, R.M. Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Garfias-Mesías, L.F.; Paul, D.R. Thermal degradation of commercially available organoclays studied by TGA–FTIR. Thermochim. Acta 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Han, L.; Han, C.; Cao, W.; Wang, X.; Bian, J.; Dong, L. Preparation and characterization of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/silica nanocomposites. Polym. Eng. Sci. 2011, 52, 250–258. [Google Scholar] [CrossRef]
- Hongchao, Z.; Kanishka, B.; Pengqun, K.; Juming, T.; Barbara, R.; Scott, M.; Shyam, S. Effects of Oxygen and Water Vapor Transmission Rates of Polymeric Pouches on Oxidative Changes of Microwave-Sterilized Mashed Potato. Food Bioprocess Technol. 2016, 9, 341–351. [Google Scholar] [CrossRef]
- Wilson, A.S. Plasticisers: Principles and Practice; The Institute of Materials: London, UK, 1995. [Google Scholar]
- Järnström, H.; Saarela, K.; Kalliokoski PPasanen, A.-L. Comparison of VOC and ammonia emissions from individual PVC materials, adhesives and from complete structures. Environ. Int. 2008, 34, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ohkado, Y.; Kawamura, Y.; Mutsuga, M.; Tamura, H.-O.; Tanamoto, K. Analysis of residual volatiles in recycled polyethylene terephthalate. J. Food Hyg. Soc. Jpn. 2005, 46, 13–20. [Google Scholar] [CrossRef]
- Hu, M.; Jacobsen, C. Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Subchapter 13.5.5 Aldehyde Scavenging Packaging; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-1-63067-056-6. [Google Scholar]
- Azarbad, M.H.; Jeleń, H. Determination of Hexanal—An Indicator of Lipid Oxidation by Static Headspace Gas Chromatography (SHS-GC) in Fat-Rich Food Matrices. Food Anal. Methods 2014, 8, 1727–1733. [Google Scholar] [CrossRef]





| Material Formulation | Commercial Matrix Used | Nature of the PHA | Type of Reinforcement (3 wt %) |
|---|---|---|---|
| PHA1005 | PHA1005 (Metabolix) | P3HB-co-P4HB | (3HB-co-17 mol % 4HB) & Talc |
| PHA1005_T1 | PHA1005 (Metabolix) | P3HB-co-P4HB | T1: Aminosilane Sepiolite |
| PHA1005_T2 | PHA1005 (Metabolix) | P3HB-co-P4HB | T2: Natural Sepiolite |
| PHA1005_T3 | PHA1005 (Metabolix) | P3HB-co-P4HB | T3: Sodium Montmorillonite-quaternary ammonium salt |
| PHA3002 | PHA3002 (Metabolix) | P3HB-co-P4HB | (3HB-co-23.5 mol % 4HB) & Talc |
| PHA3002_T1 | PHA3002 (Metabolix) | P3HB-co-P4HB | T1: Aminosilane Sepiolite |
| PHA3002_T2 | PHA3002 (Metabolix) | P3HB-co-P4HB | T2: Natural Sepiolite |
| PHA3002_T3 | PHA3002 (Metabolix) | P3HB-co-P4HB | T3: Sodium Montmorillonite-quaternary ammonium salt |
| PHB226 | PHB226 (Biomer) | P3HB | Traces of PBA and plasticizer found, & Talc |
| PHB226_T1 | PHB226 (Biomer) | P3HB | T1: Aminosilane Sepiolite |
| PHB226_T2 | PHB226 (Biomer) | P3HB | T2: Natural Sepiolite |
| PHB226_T3 | PHB226 (Biomer) | P3HB | T3: Sodium Montmorillonite-quaternary ammonium salt |
| Run | Temperature (°C) | Time (min) | NaCl (1 M) |
|---|---|---|---|
| 1 | 70 | 37.5 | 0.5 |
| 2 | 50 | 60 | 0.5 |
| 3 | 90 | 60 | 0.5 |
| 4 | 70 | 60 | 0 |
| 5 | 90 | 15 | 0.5 |
| 6 | 70 | 15 | 1 |
| 7 | 70 | 37.5 | 0.5 |
| 8 | 90 | 37.5 | 0 |
| 9 | 90 | 37.5 | 1 |
| 10 | 70 | 15 | 0 |
| 11 | 50 | 37.5 | 1 |
| 12 | 70 | 37.5 | 0.5 |
| 13 | 50 | 37.5 | 0 |
| 14 | 70 | 60 | 1 |
| 15 | 50 | 15 | 0.5 |
| 16 | 70 | 37.5 | 0.5 |
| Materials | Tonset [°C] | Tmax [°C] | T50 wt % [°C] | FR [%] | ||
|---|---|---|---|---|---|---|
| PHA1005 | 268.13 | 278.33 | 279.62 | 9.45 | ||
| PHA1005_T1 | 267.94 | 275.51 | 278.89 | 12.93 | ||
| PHA1005_T2 | 265.75 | 278.33 | 278.52 | 13.56 | ||
| PHA1005_T3 | 263.20 | 278.33 | 277.25 | 13.27 | ||
| PHA3002 | 290.01 | 307.67 | 305.34 | 8.39 | ||
| PHA3002_T1 | 287.46 | 307.67 | 305.61 | 11.71 | ||
| PHA3002_T2 | 283.63 | 302.33 | 300.23 | 10.93 | ||
| PHA3002_T3 | 284.73 | 299.67 | 298.95 | 11.26 | ||
| PHB226 | 275.97 | 386.87 | 297.00 | 403.70 | 293.84 | 2.73 |
| PHB226_T1 | 283.63 | 384.31 | 299.82 | 411.67 | 301.50 | 5.61 |
| PHB226_T2 | 278.52 | 383.03 | 297.00 | 407.30 | 296.40 | 5.83 |
| PHB226_T3 | 272.14 | 347.29 | 291.49 | 382.86 | 287.46 | 6.37 |
| Volatile Compound | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Material | 1-Hexanol | Heptanal | Octanal | Decanal | α-Methylstyrene | Benzaldehyde | ||||||
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |
| (μg/g sample) | (μg/g sample) | (μg/g sample) | (μg/g sample) | (μg/g sample) | (μg/g sample) | |||||||
| PHA 1005 | 18.8 | 3.8 | 14.8 | 6.8 | 68.6 | 14.9 | 900 | 500 | 102.8 | 35.4 | 6.4 | 0.3 |
| PHA 1005_T1 | 13.2 | 1.1 | 15.8 | 1.5 | 68.1 | 7.1 | 2100 | 500 | 283.6 | 12.2 | 5.7 | 1.2 |
| PHA 1005_T2 | 8.2 | 1.3 | 15.3 | 3.2 | 78.1 | 13.9 | 1300 | 200 | 25.7 | 10.0 | 4.3 | 0.3 |
| PHA 1005_T3 | 5.3 | 0.7 | 81.2 | 29.3 | 5.3 | 0.7 | 5300 | 700 | 89.6 | 33.5 | 5.3 | 0.7 |
| PHA 3002 | 2.7 | 1.0 | 18.8 | 10.0 | 75.4 | 18.8 | 1000 | 300 | 135.4 | 28.4 | 4.5 | 1.5 |
| PHA 3002_T1 | 2.8 | 1.0 | 27.9 | 1.7 | 41.2 | 6.5 | 3300 | 500 | 279.5 | 17.6 | 7.1 | 0.3 |
| PHA 3002_T2 | 16.6 | 2.9 | 41.4 | 16.3 | 25.3 | 7.4 | 2400 | 400 | 59.3 | 11.6 | 4.6 | 0.7 |
| PHA 3002_T3 | 8.1 | 2.7 | 98.8 | 11.1 | 53.6 | 16.8 | 1800 | 400 | nd | nd | 6.7 | 1.1 |
| PHB 226 | 3.7 | 0.9 | 4.0 | 0.6 | 26.7 | 0.5 | 2300 | 100 | 40.4 | 4.6 | 7.7 | 0.7 |
| PHB 226_T1 | 1.2 | 0.9 | 3.2 | 1.1 | 32.6 | 6.0 | 1900 | 200 | 43.4 | 7.1 | 7.2 | 2.6 |
| PHB 226_T2 | 2.3 | 0.6 | 10.3 | 0.9 | 39.3 | 7.7 | 1300 | 300 | 10.3 | 2.3 | 4.9 | 0.5 |
| PHB 226_T3 | 1.6 | 0.6 | 3.5 | 7.6 | 71.3 | 17.9 | 2800 | 700 | 39.6 | 17.3 | 6.5 | 2.7 |
| Volatile | PHA 1005 | PHA 3002 | PHB 226 | ||
|---|---|---|---|---|---|
| 1-Hexanol | T3 | T1 | T1 | ||
| Heptanal | T2 | T1 | T1 | ||
| Octanal | T3 | T2 | T1 | ||
| Decanal | T2 | T3 | T2 | ||
| α-Methylstyrene | T2 | T3 | T2 | ||
| Benzaldehyde | T2 | T2 | T2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Quiles, L.; Valdés, A.; Cuello, Á.F.; Jiménez, A.; Garrigós, M.d.C.; Castell, P. Reducing off-Flavour in Commercially Available Polyhydroxyalkanoate Materials by Autooxidation through Compounding with Organoclays. Polymers 2019, 11, 945. https://doi.org/10.3390/polym11060945
García-Quiles L, Valdés A, Cuello ÁF, Jiménez A, Garrigós MdC, Castell P. Reducing off-Flavour in Commercially Available Polyhydroxyalkanoate Materials by Autooxidation through Compounding with Organoclays. Polymers. 2019; 11(6):945. https://doi.org/10.3390/polym11060945
Chicago/Turabian StyleGarcía-Quiles, Lidia, Arantzazu Valdés, Ángel Fernández Cuello, Alfonso Jiménez, María del Carmen Garrigós, and Pere Castell. 2019. "Reducing off-Flavour in Commercially Available Polyhydroxyalkanoate Materials by Autooxidation through Compounding with Organoclays" Polymers 11, no. 6: 945. https://doi.org/10.3390/polym11060945
APA StyleGarcía-Quiles, L., Valdés, A., Cuello, Á. F., Jiménez, A., Garrigós, M. d. C., & Castell, P. (2019). Reducing off-Flavour in Commercially Available Polyhydroxyalkanoate Materials by Autooxidation through Compounding with Organoclays. Polymers, 11(6), 945. https://doi.org/10.3390/polym11060945