Polydimethylsiloxane/Nanodiamond Composite Sponge for Enhanced Mechanical or Wettability Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the PDMS/oND Composite
2.3. Fabrication of the PDMS/rND Composite
2.4. Fabrication of the PDMS/ND Composite Sponges
2.5. Measurement of Mechanical Parameters
2.6. Analysis of the Raw Materials, Composite Sponges, and Bulk Composite
3. Results and Discussion
3.1. Analysis of Oxidized and Hydrogenated ND Particles
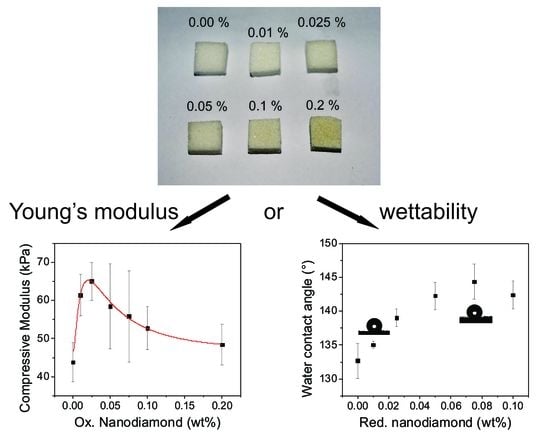
3.2. Mechanical Properties of PDMS/ND Composites
3.3. FTIR Analysis of PDMS/ND Composites
3.4. Surface Wettability of the As-Made PDMS/ND Composite Sponges
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Badamshina, E.; Gafurova, M. Polymeric nanocomposites containing non-covalently bonded fullerene C60: Properties and applications. J. Mater. Chem. 2012, 22, 9427–9438. [Google Scholar] [CrossRef]
- Rahmat, M.; Hubert, P. Carbon nanotube–polymer interactions in nanocomposites: A review. Compos. Sci. Technol. 2011, 72, 72–84. [Google Scholar] [CrossRef]
- Arjmand, M.; Chizari, K.; Krause, B.; Pötschke, P.; Sundararaj, U. Effect of synthesis catalyst on structure of nitrogen-doped carbon nanotubes and electrical conductivity and electromagnetic interference shielding of their polymeric nanocomposites. Carbon 2016, 98, 358–372. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Zhao, P.; Liu, T.; Sun, J. Titanium/nanodiamond nanocomposites: Effect of nanodiamond on microstructure and mechanical properties of titanium. Mater. Des. 2017, 131, 144–155. [Google Scholar] [CrossRef]
- Liu, M.; Xu, D.; Wang, K.; Deng, F.; Wan, Q.; Zeng, G.; Huang, Q.; Zhang, X.; Wei, Y. Nanodiamond based supermolecular nanocomposites: Preparation and biocompatibility evaluation. RSC Adv. 2015, 5, 96983–96989. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Jabeen, S.; Kausar, A.; Muhammad, B.; Gul, S.; Farooq, M. A Review on Polymeric Nanocomposites of Nanodiamond, Carbon Nanotube, and Nanobifiller: Structure, Preparation and Properties. Polym. Plast. Technol. Eng. 2015, 54, 1379–1409. [Google Scholar] [CrossRef]
- Skountzos, E.N.; Anastassiou, A.; Mavrantzas, V.G.; Theodorou, D.N. Determination of the Mechanical Properties of a Poly(methyl methacrylate) Nanocomposite with Functionalized Graphene Sheets through Detailed Atomistic Simulations. Macromolecules 2014, 47, 8072–8088. [Google Scholar] [CrossRef]
- Moradi, M.; Mohandesi, J.A.; Haghshenas, D.F. Mechanical properties of the poly(vinyl alcohol) based nanocomposites at low content of surfactant wrapped graphene sheets. Polymer 2015, 60, 207–214. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z.; Then, Y.Y.; Loo, Y.Y. Reinforcement of graphene nanoplatelets on plasticized poly(lactic acid) nanocomposites: Mechanical, thermal, morphology, and antibacterial properties. J. Appl. Polym. Sci. 2015, 132, 41652. [Google Scholar] [CrossRef]
- Yi, P.; Awang, R.A.; Rowe, W.S.T.; Kalantar-zadeh, K.; Khoshmanesh, K. PDMS nanocomposites for heat transfer enhancement in microfluidic platforms. Lab Chip 2014, 14, 3419–3426. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Li, H.; Wu, S.; Liu, Y.; Zheng, J. Enhanced mechanical and gas barrier properties of rubber nanocomposites with surface functionalized graphene oxide at low content. Polymer 2013, 54, 1930–1937. [Google Scholar] [CrossRef]
- Scherillo, G.; Lavorgna, M.; Buonocore, G.G.; Zhan, Y.H.; Xia, H.S.; Mensitieri, G.; Ambrosio, L. Tailoring Assembly of Reduced Graphene Oxide Nanosheets to Control Gas Barrier Properties of Natural Rubber Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 2230–2234. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent Progress in Fabrication and Application of Polydimethylsiloxane Sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, J.; Zhu, D.; Zheng, J.; Handschuh-Wang, S.; Zhou, X.; Zhang, J.; Liu, Y.; Liu, Z.; He, C.; et al. Hydrophilic Sponges for Leaf-Inspired Continuous Pumping of Liquids. Adv. Sci. 2017, 4, 1700028. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Huang, Y.; Zhang, T.; Liu, Y.; Yang, B.; He, C.; Zhou, X.; Zhang, J. Organic sponge photocatalysis. Green Chem. 2017, 19, 2925–2930. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, D.; Handschuh-Wang, S.; Lv, G.; Wang, J.; Li, T.; Chen, C.; He, C.; Zhang, J.; Liu, Y.; et al. Bioinspired, Mechano-Regulated Interfaces for Rationally Designed, Dynamically Controlled Collection of Oil Spills from Water. Glob. Chall. 2017, 1, 1600014. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Heo, J.-H.; Jeon, S.; Park, J.H.; Kim, S.; Kang, H.-W. Bio-inspired hollow PDMS sponge for enhanced oil–water separation. J. Hazard. Mater. 2019, 365, 494–501. [Google Scholar] [CrossRef]
- Liang, S.; Li, Y.; Chen, Y.; Yang, J.; Zhu, T.; Zhu, D.; He, C.; Liu, Y.; Handschuh-Wang, S.; Zhou, X. Liquid metal sponges for mechanically durable, all-soft, electrical conductors. J. Mater. Chem. C 2017, 5, 1586–1590. [Google Scholar] [CrossRef]
- Liang, S.; Li, Y.; Yang, J.; Zhang, J.; He, C.; Liu, Y.; Zhou, X. 3D Stretchable, Compressible, and Highly Conductive Metal-Coated Polydimethylsiloxane Sponges. Adv. Mater. Technol. 2016, 1, 1600117. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.H.; Kim, J.; Choi, S.; Lee, J.; Park, I.; Hyeon, T.; Kim, D.H. Reverse-Micelle-Induced Porous Pressure-Sensitive Rubber for Wearable Human-Machine Interfaces. Adv. Mater. 2014, 26, 4825. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Ouyang, X.; Wen, C. Flexible Superhydrophobic and Superoleophilic MoS2 Sponge for Highly Efficient Oil-Water Separation. Sci. Rep. 2016, 6, 27207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami, P.; Salkhi Khasraghi, S.; Hashemi, M.; Rabiei, S.; Shojaei, A. Polymer/nanodiamond composites - a comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019, 269, 122–151. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Ho, D.; Wang, C.-H.K.; Chow, E.K.-H. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Su, D. Fabrication of Nitrogen-Modified Annealed Nanodiamond with Improved Catalytic Activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhao, M.Q.; Chen, C.; Pentecost, A.; Maleski, K.; Mathis, T.; Zhang, X.Q.; Zhang, Q.; Jiang, J.J.; Gogotsi, Y. Nanodiamonds suppress the growth of lithium dendrites. Nat. Commun. 2017, 8, 9. [Google Scholar] [CrossRef]
- Hajiali, F.; Shojaei, A. Network structure and mechanical properties of polydimethylsiloxane filled with nanodiamond—Effect of degree of silanization of nanodiamond. Compos. Sci. Technol. 2017, 142, 227–234. [Google Scholar] [CrossRef]
- Hajiali, F.; Shojaei, A. Silane functionalization of nanodiamond for polymer nanocomposites-effect of degree of silanization. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 254–263. [Google Scholar] [CrossRef]
- Mermet-Guyennet, M.; Dinkgreve, M.; Habibi, M.; Martzel, N.; Sprik, R.; Denn, M.; Bonn, D. Dependence of nonlinear elasticity on filler size in composite polymer systems. Rheol. Acta 2017, 56, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Mochalin, V.N.; Gogotsi, Y. Nanodiamond–polymer composites. Diam. Relat. Mater. 2015, 58, 161–171. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Chang, I.P.; Hwang, K.C.; Ho, J.-a.A.; Lin, C.-C.; Hwu, R.J.R.; Horng, J.-C. Facile Surface Functionalization of Nanodiamonds. Langmuir 2010, 26, 3685–3689. [Google Scholar] [CrossRef]
- Wang, T.; Handschuh-Wang, S.; Yang, Y.; Zhuang, H.; Schlemper, C.; Wesner, D.; Schönherr, H.; Zhang, W.; Jiang, X. Controlled Surface Chemistry of Diamond/β-SiC Composite Films for Preferential Protein Adsorption. Langmuir 2014, 30, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Zuerbig, V.; Gao, F.; Hoffmann, R.; Nebel, C.E.; Ambacher, O.; Lebedev, V. Appropriate Salt Concentration of Nanodiamond Colloids for Electrostatic Self-Assembly Seeding of Monosized Individual Diamond Nanoparticles on Silicon Dioxide Surfaces. Langmuir 2015, 31, 5319–5325. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Handschuh-Wang, S.; Qin, P.; Yang, Y.; Zhou, X.; Tang, Y. Enhancing the colloidal stability of detonation synthesized diamond particles in aqueous solutions by adsorbing organic mono-, bi- and tridentate molecules. J. Colloid Interface Sci. 2017, 499, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Handschuh-Wang, S.; Zhang, S.; Zhou, X.; Tang, Y. Enhanced nucleation of diamond on three dimensional tools via stabilized colloidal nanodiamond in electrostatic self-assembly seeding process. J. Colloid Interface Sci. 2017, 506, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jee, A.-Y.; Lee, M. Surface functionalization and physicochemical characterization of diamond nanoparticles. Curr. Appl. Phys. 2009, 9, e144–e147. [Google Scholar] [CrossRef]
- Petit, T.; Girard, H.A.; Trouve, A.; Batonneau-Gener, I.; Bergonzo, P.; Arnault, J.-C. Surface transfer doping can mediate both colloidal stability and self-assembly of nanodiamonds. Nanoscale 2013, 5, 8958–8962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuard, D.; Tzvetkova-Chevolleau, T.; Decossas, S.; Tracqui, P.; Schiavone, P. Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectron. Eng. 2008, 85, 1289–1293. [Google Scholar] [CrossRef] [Green Version]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Moser, R.; Kettlgruber, G.; Siket, C.M.; Drack, M.; Graz, I.M.; Cakmak, U.; Major, Z.; Kaltenbrunner, M.; Bauer, S. From Playroom to Lab: Tough Stretchable Electronics Analyzed with a Tabletop Tensile Tester Made from Toy-Bricks. Adv. Sci. 2016, 3, 1500396. [Google Scholar] [CrossRef]
- Gavrilov, A.S.; Voznyakovskii, A.P. Rheological characteristics and relaxation properties of polymer-nanodiamond composites. Russ. J. Appl. Chem. 2009, 82, 1041–1045. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Q.; Wang, H. Synthesis and Characterization of Nanodiamond Reinforced Chitosan for Bone Tissue Engineering. J. Funct. Biomater. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Lamberti, A.; Virga, A.; Rivolo, P.; Angelini, A.; Giorgis, F. Easy Tuning of Surface and Optical Properties of PDMS Decorated by Ag Nanoparticles. J. Phys. Chem. B 2015, 119, 8194–8200. [Google Scholar] [CrossRef]
- Chen, I.J.; Lindner, E. The Stability of Radio-Frequency Plasma-Treated Polydimethylsiloxane Surfaces. Langmuir 2007, 23, 3118–3122. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Xu, K.-Q.; Tang, C.-Y.; Lau, W.-m.; Yin, C.-B.; Zhu, Y.; Mei, J.; Lee, J.; Hui, D.; Nie, H.-Y.; et al. Cross-Linking the Surface of Cured Polydimethylsiloxane via Hyperthemal Hydrogen Projectile Bombardment. ACS Appl. Mater. Interfaces 2015, 7, 8515–8524. [Google Scholar] [CrossRef]
- Gallas, J.-P.; Goupil, J.-M.; Vimont, A.; Lavalley, J.-C.; Gil, B.; Gilson, J.-P.; Miserque, O. Quantification of Water and Silanol Species on Various Silicas by Coupling IR Spectroscopy and in-Situ Thermogravimetry. Langmuir 2009, 25, 5825–5834. [Google Scholar] [CrossRef]
- Wang, D.H.; Tan, L.-S.; Huang, H.; Dai, L.; Ōsawa, E. In-Situ Nanocomposite Synthesis: Arylcarbonylation and Grafting of Primary Diamond Nanoparticles with a Poly(ether−ketone) in Polyphosphoric Acid. Macromolecules 2009, 42, 114–124. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, T.; Li, Y.; Huang, L.; Handschuh-Wang, S. Polydimethylsiloxane/Nanodiamond Composite Sponge for Enhanced Mechanical or Wettability Performance. Polymers 2019, 11, 948. https://doi.org/10.3390/polym11060948
Zhao X, Wang T, Li Y, Huang L, Handschuh-Wang S. Polydimethylsiloxane/Nanodiamond Composite Sponge for Enhanced Mechanical or Wettability Performance. Polymers. 2019; 11(6):948. https://doi.org/10.3390/polym11060948
Chicago/Turabian StyleZhao, Xuxin, Tao Wang, Yaoyao Li, Lei Huang, and Stephan Handschuh-Wang. 2019. "Polydimethylsiloxane/Nanodiamond Composite Sponge for Enhanced Mechanical or Wettability Performance" Polymers 11, no. 6: 948. https://doi.org/10.3390/polym11060948
APA StyleZhao, X., Wang, T., Li, Y., Huang, L., & Handschuh-Wang, S. (2019). Polydimethylsiloxane/Nanodiamond Composite Sponge for Enhanced Mechanical or Wettability Performance. Polymers, 11(6), 948. https://doi.org/10.3390/polym11060948