Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Unloaded Nanofibrous Scaffolds Preparation
2.2.2. Unloaded Nanofibrous Scaffold Degradation via Lysozyme
2.2.3. AgNP Nanofibrous Scaffolds Preparation
Synthesis of Silver Nanoparticles (AgNPs)
Electrospinning Process
2.2.4. AgNP Nanofibrous Scaffolds Characterizations
Chemico-Physical Characterization
In Vitro Cells Adhesion and Proliferation Assay
In Vitro Antimicrobial Assay
Statistical Analysis
3. Results and Discussion
3.1. Nanofibrous Scaffolds Degradation Via Lysozyme
3.2. AgNP Nanofibrous Scaffolds Chemico-Physical Characterization
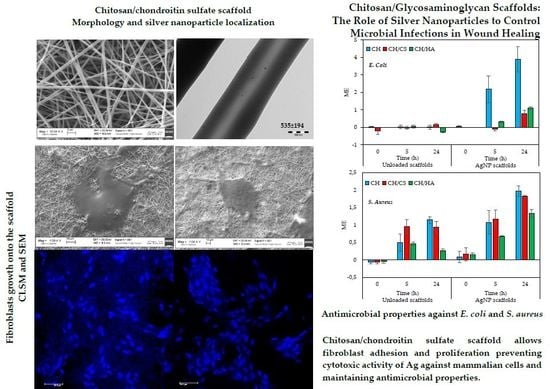
3.3. In Vitro Cells Adhesion and Proliferation
3.4. In Vitro Antimicrobial Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoes, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendoca, A.G.; Correira, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Zewde, B.; Ambaye, A.; Stubbs, J.; Raghavan, D. A review of stabilized Silver Nanoparticles–synthesis, Biological properties, characterization and potential areas of applications, JMS Nanotechnol. Nanomed 2016, 4, 1–14. Available online: https://pdfs.semanticscholar.org/9f18/5dd6833150dd56d853084861adc90b061851.pdf (accessed on 18 July 2019).
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; De La Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; Van Loveren, H.; De Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, T.C.; Matthews, P.; Ahmad, R. Dry-heat sterilization of parenteral oil vehicles. Int. J. Pharm. Compd. 2000, 4, 223–224. [Google Scholar] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Aguzzi, C.; Mori, M.; Grisoli, P.; Cerezo, P.; Tenci, M.; Viseras, C.; et al. Montmorillonite-chitosan-silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohydr. Polym. 2014, 102, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Taglietti, A.; Dacarro, G.; Diaz-Fernandez, Y.A.; Galli, M.; Grisoli, P.; Patrini, M.; Santucci De Magistris, G.; Zanoni, R. Self-assembled monolayers of silver nanoparticles firmly grafted on glass surfaces: Low Ag+ release for an efficient antibacterial activity. J. Colloid Interface Sci. 2010, 350, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Del Fante, C.; Vigani, B.; Black, L.; Ferrari, F. Electrospun gelatin–chondroitin sulfate scaffolds loaded with platelet lysate promote immature cardiomyocyte proliferation. Polymers 2018, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze dried chitosan acetate dressings with glycosaminoglycans and traxenamic acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Marciello, M.; Sandri, G.; Ferrari, F.; Bonferoni, M.C.; Papetti, A.; Caramella, C.; Dacarro, C.; Grisoli, P. Wound dressings based on chitosans and hyaluronic acid for the release of chlorhexidine diacetate in skin ulcer therapy. Pharm. Dev. Technol. 2007, 12, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Russell, Hugo Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization, 6th ed.; Fraise, A.P.; Lambert, P.A.; Masillard, J.Y. (Eds.) Blackwell Publishing: Hoboken, NJ, USA, 2004. [Google Scholar]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.J.; Su, W.H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. Available online: https://jfoodprotection.org/doi/pdf/10.4315/0362-028X-62.3.239 (accessed on 18 July 2019). [CrossRef] [PubMed]








| AgNPs Loaded | Unloaded | ||||||
|---|---|---|---|---|---|---|---|
| % w/w | P | CH | CA | CS | HA | AgNPs/Acetic acid | Water/Acetic acid |
| CH | 10 | 2.5 | 2.5 | -- | -- | 55/45 | 55/45 |
| CH/CS | 10 | 2.5 | 2.5 | 0.5 | -- | 55/45 | 55/45 |
| CH/HA | 10 | 2.5 | 2.5 | -- | 0.5 | 55/45 | 55/45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. https://doi.org/10.3390/polym11071207
Sandri G, Miele D, Faccendini A, Bonferoni MC, Rossi S, Grisoli P, Taglietti A, Ruggeri M, Bruni G, Vigani B, et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers. 2019; 11(7):1207. https://doi.org/10.3390/polym11071207
Chicago/Turabian StyleSandri, Giuseppina, Dalila Miele, Angela Faccendini, Maria Cristina Bonferoni, Silvia Rossi, Pietro Grisoli, Angelo Taglietti, Marco Ruggeri, Giovanna Bruni, Barbara Vigani, and et al. 2019. "Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing" Polymers 11, no. 7: 1207. https://doi.org/10.3390/polym11071207
APA StyleSandri, G., Miele, D., Faccendini, A., Bonferoni, M. C., Rossi, S., Grisoli, P., Taglietti, A., Ruggeri, M., Bruni, G., Vigani, B., & Ferrari, F. (2019). Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers, 11(7), 1207. https://doi.org/10.3390/polym11071207