A Method to Improve the Characteristics of EPDM Rubber Based Eco-Composites with Electron Beam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Experimental Installation and Sample Irradiation
2.3. Laboratory Tests
2.3.1. Mechanical Characteristics
2.3.2. Cross-Linking Evaluation
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.4. Water Uptake Evaluation
2.3.5. Rubber-Filler Interaction
3. Results and Discussion
3.1. Mechanical Characteristics
3.2. Gel Fraction and Cross-Link Densities
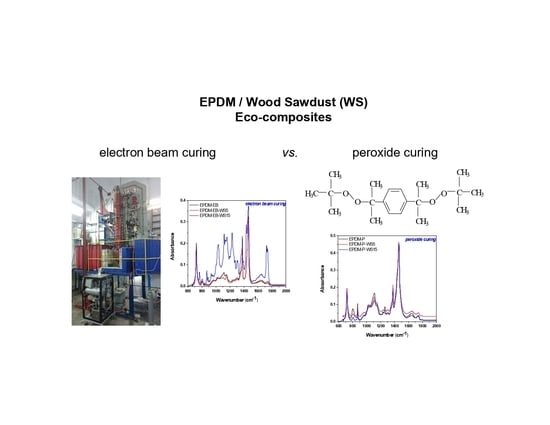
3.3. FTIR Analysis
3.4. Water Uptake Test Results
3.5. Rubber-Filler Interaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radi, H.; Mousaa, I.M. Characterization Study of EPDM Rubber Vulcanized by Gamma Radiation in The Presence of Epoxidized Soybean Oil. Egypt. J. Radiat. Sci. Appl. 2015, 28, 121–134. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, S.W.; Park, E.S.; Hwang, J.Y.; Lee, D.S. High-Performance Adhesives Based on Maleic Anhydride-g-EPDM Rubbers and Polybutene for Laminating Cast Polypropylene Film and Aluminum Foil. Coatings 2019, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Kruzelak, J.; Sykora, R.; Hudec, I. Vulcanization of Rubber Compounds with Peroxide Curing Systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Quirk, R.P. Overview of Curing and Cross-linking of Elastomers. Prog. Rubber Plast. Technol. 1988, 4, 31–45. [Google Scholar]
- Samarzija-Jovanovic, S.; Jovanovic, V.; Cincovic, M.M.; Simendic, J.B.; Markovic, G. Comparative study of radiation effect on rubber—Carbon black compounds. Compos. Part B 2014, 62, 183–190. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Fifere, N.; Craciun, G.; Varganici, C.; Doroftei, F. Exploring the Effect of Electron Beam Irradiation on the Properties of Some EPDM-Flax Fiber Composites. Polym. Compos. 2019, 40, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Ighigeanu, D. Wood Sawdust/Natural Rubber Ecocomposites Cross-Linked by Electron Beam Irradiation. Materials 2016, 9, 503. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Dumitrascu, M. New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci. World J. 2014, 684047. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Zuga, N. Crosslinking and grafting ethylene vinyl acetate copolymer with accelerated electrons in the presence of polyfunctional monomers. Polym. Bull. 2012, 68, 263–285. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G. Vulcanization of ethylene-propyleneterpolymer-based rubber mixtures by radiation processing. J. Appl. Polym. Sci. 2013, 128, 2325–2336. [Google Scholar] [CrossRef]
- Czvikovszky, T. Degradation effects in polymers, in IAEA-TECDOC-1420, Advances in radiation chemistry of polymers. In Proceedings of the Technical Meeting, Notre Dame, IN, USA, 13–17 September 2003; pp. 91–102. [Google Scholar]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Chirila, C. Development and Characterization of Polymer Eco-Composites Based on Natural Rubber Reinforced with Natural Fibers. Materials 2017, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Craciun, G.; Manaila, E.; Stelescu, M.D. New Elastomeric Materials Based on Natural Rubber Obtained by Electron Beam Irradiation for Food and Pharmaceutical Use. Materials 2016, 9, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aziz, M.M.; Basfar, A.A. Thermal Stability of Radiation Vulcanized EPDM Rubber. In Proceedings of the 8th Arab International Conference on Polymer Science and Technology, Cairo-Sharm El-Shiekh, Egypt, 27–30 November 2005. [Google Scholar]
- Clough, R.L. Isotopic exchange in gamma-irradiated mixtures of C24H50 and C24D50: Evidence of free radical migration in the solid state. J. Chem. Phys. 1987, 87, 1588–1595. [Google Scholar] [CrossRef]
- Gatos, K.G.; Karger-Kocsis, J. Effects of primary and quaternary amine intercalants on the organoclay dispersion in a sulphur-cured EPDM rubber. Polymer 2005, 46, 3069–3076. [Google Scholar] [CrossRef]
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press: Oxford, UK, 1960. [Google Scholar]
- Dole, M. (Ed.) The Radiation Chemistry of Macromolecules; Academic Press: New York, NY, USA, 1972; Volume I. [Google Scholar]
- Grassia, N.; Scott, G. Polymer Degradation and Stabilization; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- El-Zayat, M.M.M. Radiation Curing of Rubber/Thermoplastic Composites Containing Different Inorganic Fillers. Master’s Thesis, Menoufia University, Shibin Al Kawm, Egypt, 2012. [Google Scholar]
- Planes, E.; Chazeau, L.; Vigier, G.; Fournier, J.; Stevenson-Royaud, I. Influence of fillers on mechanical properties of ATH filled EPDM during ageing by gamma irradiation. Polym. Degrad. Stab. 2010, 95, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Chaiear, N. Report No. 138 Health and safety in the rubber industry. In Rapra Review Reports; Rapra Technology Limited: Shrewsbury, UK, 2001; Volume 12, pp. 21–22. ISBN 1-85957-301-0. [Google Scholar]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interfacial Properties of Mixed DPPC–Hydrophobic Fumed Silica Nanoparticle Layers. J. Phys. Chem. C 2015, 119, 21024–21034. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Zabiegaj, D.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of Carbon Black Particles and Dipalmitoylphosphatidylcholine at the Water/Air Interface: Thermodynamics and Rheology. J. Phys. Chem. C 2015, 119, 26937–26947. [Google Scholar] [CrossRef]
- Lopez-Manchado, M.A.; Biagiotti, J.; Arroyo, M.; Kenny, J.M. Ternary composites based on PP-EPDM blends reinforced with flax fibers. Part I: Processing and thermal behavior. Polym. Eng. Sci. 2003, 43, 1018–1030. [Google Scholar] [CrossRef]
- Rozik, N.N.; Abd-El.Messieh, S.L.; Yaseen, A.A.; Abd-El-Hafiz, A.S. Dielectric and mechanical properties of natural nanofibers—Reinforced ethylene propylene diene rubber: Carrot foliage and corn gluten. Polym. Eng. Sci. 2013, 53, 874–881. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M. Green composites: A brief review. Compos. Part A 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Gaceva, G.B.; Avella, M.; Malinconico, M.; Buzarovska, A.; Grozdanov, A.; Gentile, G.; Errico, M.E. Natural Fiber Eco-Composites. Polym. Compos. 2007, 28, 98–107. [Google Scholar] [CrossRef]
- Cristaldi, G.; Latteri, A.; Recca, G.; Cicala, G. Composites Based on Natural Fibre Fabrics. In Woven Fabric Engineering; Dubrovski, P.D., Ed.; IntechOpen: Rijeka, Croatia, 2010; pp. 318–342. Available online: http://www.intechopen.com (accessed on 2 June 2014).
- Begum, K.; Islam, M.A. Natural fiber as a substitute to synthetic fiber in Polymer Composites: A Review. Res. J. Eng. Sci. 2013, 2, 46–53. [Google Scholar]
- Nora’asheera, M.N. Composites From Polypropylene (PP) Reinforced With Oil Palm Empty Fruit Bunch (OPEFB) Fibre. Bachelor’s Thesis, University of Malaysia Pahang, Pahang, Malaysia, 2011. [Google Scholar]
- Fakhrul, T.; Mahbub, R.; Islam, M.A. Properties of wood sawdust and wheat Flour Reinforced Polypropylene Composites. J. Mod. Sci. Technol. 2013, 1, 135–148. [Google Scholar]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Fiti, M. Dozimetria Chimica a Radiatiilor Ionizante (Ionizing Radiation Chemical Dosimetry), 1st ed.; Editura Academiei Republicii Socialiste Romania: Bucuresti, Romania, 1973; pp. 24–70. [Google Scholar]
- Cleland, M.R. Industrial Applications of Electron Accelerators—Ion Beam Applications. Presented at the CERN Accelerator School/Small Accelerator Course, Zeegse, The Netherlands, 24 May–2 June 2005. [Google Scholar]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C.; Pamfil, D.; Doroftei, F. Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers 2018, 10, 1206. [Google Scholar] [CrossRef] [Green Version]
- Anelli, P.; Baccaro, S.; Carenza, M.; Palma, G. Radiation grafting of hydrophilic monomers onto ethylenepropylene rubber. Radiat. Phys. Chem. 1995, 46, 1031–1035. [Google Scholar] [CrossRef]
- Nashar, K.; Gosh, U.; Heinrich, G. Influence of molecular structure of blend components on the performance of thermoplastic vulcanisates prepared in electron induced reactive processing. Polymer 2016, 91, 204–210. [Google Scholar] [CrossRef]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Mathew, L.; Ulahannan, J.; Joseph, R. Effect of curing temperature, fibre loading and bonding agent on the equilibrium swelling of isora-natural rubber composites. Compos. Interfaces 2006, 13, 391–401. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, M.; Jia, D.; Zhou, Y. Synthesis of natural rubber-g-maleic anhydride and its use as a compatibilizer in natural rubber/short nylon fiber composites. Chin. J. Polym. Sci. 2013, 31, 1127–1138. [Google Scholar] [CrossRef]
- Planes, E.; Chazeau, L.; Vigier, G.; Fournier, J. Evolution of EPDM networks aged by gamma irradiation—Consequence on the mechanical properties. Polymer 2009, 50, 4028–4038. [Google Scholar] [CrossRef] [Green Version]
- O’Donell, J.; Whittaker, A.K. The radiation crosslinking and scission of ethylene-propylene copolymer studied by solid-state nuclear magnetic resonance. Br. Polym. J. 1985, 17, 51–55. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Aspects regarding radiation crosslinking of elestomers. In Advanced Elastomers—Technology, Properties and Applications; Boczkowska, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–34. ISBN 978-9535107392. [Google Scholar]
- Palm, A.; Smith, J.; Driscoll, M.; Smith, L.; Scott Larsen, L. Chemical constituent influence on ionizing radiation treatment of a wood–plastic composite. Radiat. Phys. Chem. 2016, 124, 164–168. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C. Property correlations for composites based on ethylene propylene diene rubber reinforced with flax fibers. Polym. Test. 2017, 59, 75–83. [Google Scholar] [CrossRef]
- Zhao, J.; Ghebremeskel, G.N. A review of some of the factors affecting fracture and fatigue in SBR and BR vulcanizates. Rubber Chem. Technol. 2001, 74, 409–427. [Google Scholar] [CrossRef]
- Ismail, H.; Shuhelmy, S.; Edgham, M.R. The effect of a silane coupling agent on curing characteristics and mechanical properties of bamboo fibre filled NR composites. Eur. Polym. J. 2002, 38, 39–45. [Google Scholar] [CrossRef]
- Kohls, D.J.; Beauage, G. Rational design of reinforced rubber. Curr. Opin. Solid State Mater. Sci. 2002, 6, 183–194. [Google Scholar] [CrossRef]
- Ahmed, K. Hybrid composites prepared from Industrial waste: Mechanical and swelling behavior. J. Adv. Res. 2015, 6, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Kukle, S.; Gravitis, J.; Putnina, A.; Stikute, A. The effect of steam explosion treatment on technical hemp fibres. In Proceedings of the 8th International Scientific and Practical Conference, Rezekne, Latvia, 20–22 June 2011; Volume 1, pp. 230–237. [Google Scholar]
- Naskar, K. Dynamically Vulcanized PP/EPDM Thermoplastics Elastomers: Exploring Novel Routes for Crosslinking with Peroxides. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2004. [Google Scholar]
- Alvarez Grima, M.M. Novel Co-Agents for Improved Properties in Peroxide Cure of Saturated Elastomers. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2007. [Google Scholar]
- Dluzneski, P.R. Peroxide Vulcanization of Elastomers. Rubber Chem. Technol. 2001, 74, 451–492. [Google Scholar] [CrossRef]
- Moad, G. The synthesis of polyolefin graft copolymers by reactive extrusion. Prog. Polym. Sci. 1999, 24, 81–142. [Google Scholar] [CrossRef]
- Vroomen, G.L.M.; Visser, G.W.; Gehring, J. Electron Beam curing of EPDM. Rubber World 1991, 205, 23–26. [Google Scholar]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Cheng, K. Effect of crosslink density on some properties of electron beam-irradiated styreneebutadiene rubber. Radiat. Phys. Chem. 2009, 78, 1001–1005. [Google Scholar] [CrossRef]
- Majumder, P.S.; Bhowmick, A.K. Structure-property relationship of electronbeam-modified EPDM rubber. J. Appl. Polym. Sci. 2000, 77, 323–337. [Google Scholar] [CrossRef]
- Vijayabaskar, V.; Tikku, V.K.; Bhowmick, K.A. Electron beam modification and crosslinking: Influence of nitrile and carboxyl contents and level of unsaturation on structure and properties of nitrile rubber. Radiat. Phys. Chem. 2006, 75, 779–792. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.E. Analysis of EPDM Terpolymers by Near-Infrared Spectroscopy and Multivariate Calibration Methods. Appl. Spectrosc. 1989, 43, 1435–1443. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta A 2007, 68, 323–330. [Google Scholar] [CrossRef]
- Majumder, P.S.; Bhowmick, A.K. Surface and bulk-properties of EPDM rubber modified by electron beam irradiation. Radiat. Phys. Chem. 1998, 53, 63–78. [Google Scholar] [CrossRef]
- Van Gisbergen, J.G.M.; Meijer, H.E.M.; Lemstra, P.J. Structural polymer blends: 2. Processing of polypropylene, EPDM blends: Controlled rheology and morphology fixation via electron beam irradiation. Polymer 1989, 30, 2153–2157. [Google Scholar] [CrossRef] [Green Version]
- Kumutha, K.; Alias, Y. FTIR spectra of plasticized grafted natural rubber–LiCF3SO3 electrolytes. Spectrochim. Acta A 2006, 64, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Barra, G.M.O.; Crespo, J.S.; Bertolino, J.R.; Soldi, V.; Nunes Pires, A.T. Maleic Anhydride Grafting on EPDM: Qualitative and Quantitative Determination. J. Braz. Chem. Soc. 1999, 10, 31–34. [Google Scholar] [CrossRef]
- Vieira, I.; Severgnini, V.L.S.; Mazera, D.J.; Soldi, M.S.; Pinheiro, E.A.; Pires, A.T.N.; Soldi, V. Effects of maleated ethylene propylene diene rubber (EPDM) on the thermal stability of pure polyamides, and polyamide/EPDM and polyamide/poly(ethylene terephthalate) blends: Kinetic parameters and reaction mechanism. Polym. Degrad. Stab. 2001, 74, 151–157. [Google Scholar] [CrossRef]
- Schmidt, V.; Domenech, S.C.; Soldi, M.S.; Pinheiro, E.A.; Soldi, V. Thermal stability of polyaniline/ethylene propylene diene rubber blends prepared by solvent casting. Polym. Degrad. Stab. 2004, 83, 519–527. [Google Scholar] [CrossRef]
- Aarthi, R.; Ramalingam, S.; Periandy, S.; Senthil Kannan, K. Molecular structure-associated pharmacodynamic investigation on benzoyl peroxide using spectroscopic and quantum computational tools. J. Taibah Univ. Sci. 2018, 12, 104–122. [Google Scholar] [CrossRef]
- Horisawa, S.; Sunagawa, M.; Tamai, Y.; Matsuoka, Y.; Miura, T.; Terazawa, M. Biodegradation of nonlignocellulosic substances II: Physical and chemical properties of sawdust before and after use as artificial soil. J. Wood Sci. 1999, 45, 492–497. [Google Scholar] [CrossRef]
- Postek, M.T.; Poster, D.L.; Vladár, A.E.; Driscoll, M.S.; Laverne, J.A.; Tsinas, Z.I.; Al-Sheikhly, M.I. Ionizing Radiation Processing and its Potential in Advancing Biorefining and Nanocellulose Composite Materials Manufacturing. Radiat. Phys. Chem. 2017, 143, 47–52. [Google Scholar] [CrossRef]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada (McGraw-Hill Series in Forest Resources), Subsequent ed.; McGraw-Hill College: New York, NY, USA, 1980; pp. 120–125. ISBN1 10-0070484414. ISBN2 13-978-0070484405. [Google Scholar]
- Sjöström, E. Wood Chemistry: Fundamental and Applications, 2nd ed.; Academic Press, Inc.: London, UK; San Diego, CA, USA, 1993; pp. 1–20. ISBN 9780080925899. [Google Scholar]
- Driscoll, M.; Stipanovic, A.J.; Winter, W.T.; Cheng, K.; Manning, M.; Spiese, J.; Galloway, R.; Cleland, M.R. Electron beam irradiation of cellulose. Radiat. Phys. Chem. 2009, 78, 539–542. [Google Scholar] [CrossRef]
- Cheng, K.; Barber, V.A.; Driscoll, M.S.; Winter, W.T.; Stipanovic, A.J. Reducing Woody Biomass Recalcitrance by Electron Beams, Biodelignification and Hot-Water Extraction. J. Bioprocess. Eng. Biorefin. 2013, 2, 143–152. [Google Scholar] [CrossRef]
- Smith, S.; Bergey, N.S.; Salamanca-Cardona, L.; Stipanovic, A.; Driscoll, M. Electron Beam Pretreatment of Switchgrass to Enhance Enzymatic Hydrolysis to Produce Sugars for Biofuels. Carbohydr. Polym. 2014, 100, 195–201. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Matei, A.; Manciulea, I. Composites acrylic copolymers-wood waste. In Proceedings of the 4th International Conference of Advanced Composite Materials Engineering (COMAT 2012), Brasov, Romania, 18–20 October 2012; DERC Publishing House: Tewksbury, MA, USA, 2012; pp. 597–600. [Google Scholar]
- Bodirlau, R.; Teaca, C.A. Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom. J. Phys. 2009, 54, 93–104. [Google Scholar]
- Khan, M.A.; Rahaman, M.S.; Al-Jubayer, A.; Islam, J.M.M. Modification of jute fibers by radiation-induced graft copolymerization and their applications. In Cellulose-Based Graft Copolymers: Structure and Chemistry; Thakur, V.K., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 209–235. ISBN 9780429156878. [Google Scholar]
- Owen, N.L.; Thomas, D.W. Infrared studies of “hard” and “soft” woods. Appl. Spectrosc. 1989, 43, 451–455. [Google Scholar] [CrossRef]
- Hergert, H.L. Infrared spectra. In Lignins: Occurrence, Formation, Structure and Reactions; Sarkanen, K.V., Ludwig, C.H., Eds.; John Wiley & Suns Inc.: New York, NY, USA, 1971; pp. 267–297. [Google Scholar]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. FT-IR imaging microscopy to localise and characterise simultaneous and selective white-rot decay within spruce wood cells. Holzforschung 2011, 65, 411–420. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, S.; Chakraborty, R.; Bhattacharya, P. Optimization of biodegradation of natural fiber (Chorchorus capsularis): HDPE composite using response surface methodology. Iran. Polym. J. 2013, 22, 865–875. [Google Scholar] [CrossRef]
- Idrus, M.A.M.M.; Hamdan, S.; Rahman, M.R.; Islam, M.S. Treated Tropical Wood Sawdust-Polypropylene Polymer Composite: Mechanical and Morphological Study. J. Biomater. Nanobiotechnol. 2011, 2, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Gao, Y.; Wu, G.; Zhang, L. Nanocomposites of poly (lactic acid) reinforced with cellulose nanofibrils. BioResources 2010, 5, 1811–1823. [Google Scholar]
- Luo, S.; Cao, J.; Wang, X. Investigation of the interfacial compatibility of PEG and thermally modified wood flour/polypropylene composites using the stress relaxation approach. BioResources 2013, 8, 2064–2073. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; Wang, X. Properties of PEG/thermally modified wood flour/polypropylene (PP) composites. For. Stud. China 2012, 14, 307–314. [Google Scholar] [CrossRef]
- Desmet, G. Functionalization of Cotton-Cellulose Via High Energy Irradiation Initiated Grafting and Cyclodextrin Immobilization. Master’s Thesis, Faculty of Engineering University of Ghent, Ghent, Belgium, 2010. [Google Scholar]
- Nallasamy, P.; Mohan, S. Vibrational Spectra of CIS-1,4-Polyisoprene. Arab. J. Sci. Eng. 2004, 29, 17–26. [Google Scholar]
- Dubey, K.A.; Bhardwaj, Y.K.; Chaudhari, C.V.; Kumar, V.; Goel, N.K.; Sabharwal, S. Radiation processed ethylene vinyl acetate-multiple walled carbon nanotube nano-composites: Effect of MWNT addition on the gel content and crosslinking density. Express Polym. Lett. 2009, 3, 492–500. [Google Scholar] [CrossRef]
- Charlesby, A.; Pinner, S.H. Analysis of the solubility behaviour of irradiated polyethylene and other polymers. Proc. R. Soc. A 1959, 249, 367–386. [Google Scholar] [CrossRef]
- Sharifa, J.; Yunus, W.M.Z.W.; Dahlan, K.Z.H.M.; Ahmad, M.H. Preparation and properties of radiation crosslinked natural rubber/clay nanocomposites. Polym. Test. 2005, 24, 211–217. [Google Scholar] [CrossRef]
- Zagorski, Z.P. EB-crosslinking of elastomers, how does it compare with radiation crosslinking of other polymers? Radiat. Phys. Chem. 2004, 71, 261–265. [Google Scholar] [CrossRef]












| Band Position in the EPDM-P-S/EPDM-EB-S Composites (cm−1) | Functional Group |
|---|---|
| 3360–3390, 3598, 3636, 3637 (EPDM-EB-S 15) | O–H stretching vibration (3300–4000 cm−1) from wood sawdust/cellulose [62] |
| 2918 | C–H stretching vibration (2800–3000 cm−1) from EPDM [64,65,66,67] |
| 2850 (EPDM-P-S, EPDM-EB-S) | C–H stretching vibration (2800–3000 cm−1) from EPDM [64,65,66,67] and wood sawdust (polysaccharides/cellulose) [62] |
| 1775 cm−1 (EPDM-P-S) | C=O stretching vibration in P [71] |
| 1730–1740 (EPDM-P-S, EPDM-EB-S) | Aromatic skeletal vibrations caused by holocellulose (wood sawdust) [82] C=O stretch in non-conjugated ketones, carbonyls, and ester groups [82,83] |
| 1640 | C=C stretching vibration (1630 cm−1) from EPDM [68,69,70] |
| 1642–1646 (EPDM-EB-S) | Typical bands assigned to cellulose—Vibration of water molecules absorbed in cellulose [62] |
| 1539, 1540 (EPDM-P-S) | Aromatic skeletal vibrations caused by lignin (wood sawdust) [82] C=O stretch in non-conjugated ketones, carbonyls, and in ester groups [82,83] |
| 1460 | CH2 bending and rocking vibrations from EPDM [64,65,66,67] |
| 1435, 1436 (EPDM-EB-S) | Stretching and bending vibrations of –CH2 bonds, associated with the amount of the crystalline structure of the cellulose [62,84,85] |
| 1376 | CH3 bending vibration from EPDM [64,65,66,67] |
| 1034 (EPDM-EB-S) | Stretching and bending vibrations of –OH bonds in cellulose [62,84,85] |
| 1300–1050 cm−1 (EPDM-P-S) | C–O stretching vibrations emphasizing its domination over the O–O bond in P [71] |
| 950–800 cm−1 (EPDM-P-S) | O–O stretching vibration in P [71] |
| 930 (EPDM-EB-S) | Assigned to the amorphous region in cellulose [62,86] |
| 905 (EPDM-EB-S) | C–O bonds in cellulose [40,84,85] |
| 720 | CH2 bending and rocking vibrations from EPDM [64,65,66,67] |
| Irradiation Dose | Samples | Vrf | Vro/Vrf |
|---|---|---|---|
| 75 kGy | EPDM-EB-S 5 | 0.1912 | 0.8609 |
| EPDM-EB-S 15 | 0.1516 | 1.0862 | |
| 150 kGy | EPDM-EB-S 5 | 0.2920 | 0.7787 |
| EPDM-EB-S 15 | 0.2964 | 0.7670 | |
| 300 kGy | EPDM-EB-S 5 | 0.2242 | 1.0760 |
| EPDM-EB-S 15 | 0.2358 | 1.0232 | |
| 600 kGy | EPDM-EB-S 5 | 0.3401 | 0.8922 |
| EPDM-EB-S 15 | 0.3202 | 0.9478 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craciun, G.; Manaila, E.; Ighigeanu, D.; Stelescu, M.D. A Method to Improve the Characteristics of EPDM Rubber Based Eco-Composites with Electron Beam. Polymers 2020, 12, 215. https://doi.org/10.3390/polym12010215
Craciun G, Manaila E, Ighigeanu D, Stelescu MD. A Method to Improve the Characteristics of EPDM Rubber Based Eco-Composites with Electron Beam. Polymers. 2020; 12(1):215. https://doi.org/10.3390/polym12010215
Chicago/Turabian StyleCraciun, Gabriela, Elena Manaila, Daniel Ighigeanu, and Maria Daniela Stelescu. 2020. "A Method to Improve the Characteristics of EPDM Rubber Based Eco-Composites with Electron Beam" Polymers 12, no. 1: 215. https://doi.org/10.3390/polym12010215
APA StyleCraciun, G., Manaila, E., Ighigeanu, D., & Stelescu, M. D. (2020). A Method to Improve the Characteristics of EPDM Rubber Based Eco-Composites with Electron Beam. Polymers, 12(1), 215. https://doi.org/10.3390/polym12010215