Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparations
2.2. Methods
3. Results and Discussion
3.1. XRPD Analysis
3.2. FTIR Analysis
3.3. MALDI TOF Analysis
3.4. Adsorption isotherms—BET Analysis
3.5. TGA-DTA Analysis
3.6. SEM Analysis
3.7. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alves, H.J.; Junior, C.B.; Niklewicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Arau’jo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Galvagno, A.; Chiodo, V.; Urbani, F.; Freni, F. Biogas as hydrogen source for fuel cell applications. Int. Hydrog. Energy 2013, 38, 3913–3920. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Wang, W.; Gao, J.; Liu, W.; Chen, C. Partial oxidation of methane in a Zr0.84Y0.16O1.92eLa0.8Sr0.2Cr0.5Fe0.5O3-d hollow fiber membrane reactor targeting solid oxide fuel cell applications. J. Power Sources 2012, 217, 287–290. [Google Scholar] [CrossRef]
- Takagi, Y.; Kerman, K.; Ko, C.; Ramanathan, S. Operational characteristics of thin film solid oxide fuel cells with ruthenium anode in natural gas. J. Power Sources 2011, 243, 1–9. [Google Scholar] [CrossRef]
- Hao, X.; Liy, Y.; Wang, Z.; Qiao, J.; Sun, K. A novel sintering method to obtain fully dense gadolinia doped ceria by applying a direct current. J. Power Sources 2012, 210, 86–91. [Google Scholar] [CrossRef]
- Waldhäusl, J.; Preis, W.; Sitte, W. Electrochemical characterization of gadolinia- doped ceria using impedance spectroscopy and dc-polarization. Solid State Ion. 2012, 225, 453–456. [Google Scholar] [CrossRef]
- Gurel, T.; Eryigit, R. Ab initio pressure-depend vibrational and dielectric properties of CeO2. Phys. Rev. B 2006, 74, 014302. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of Ceria and CeO2-Containing Materials. Catal. Rev.-Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Spaneir, J.E.; Robinson, R.D.; Zhang, F.; Chen, S.; Herman, I.P. Size-dependent properties of CeO2-y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef] [Green Version]
- Atcison, A. Chemically-induced stresses in gadolinium-doped ceria solid oxide fuel cell electrolytes. Solid State Ion. 1997, 95, 249–258. [Google Scholar]
- Wang, S.; Wang, W.; Zuo, J.; Qian, Y. Study of the Raman spectrum of CeO2 nanometer thin films. Mater. Chem. Phys. 2001, 68, 246–248. [Google Scholar] [CrossRef]
- Bošković, S.B.; Matović, B.Z.; Vlajić, M.D.; Kristić, V.D. Modified glycine nitrate procedure (MGNP) for the synthesis of SOFC nano-powders. Ceram. Int. 2007, 33, 89–93. [Google Scholar] [CrossRef]
- Bošković, S.; Đurović, D.; Dohčević-Mitrović, Z.; Popović, Z.; Zinkevich, M.; Aldinger, F. Self Propagating room temperature synthesis of nano-powders for SOFC. J. Power Sources 2005, 145, 237–242. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Murashkina, A.A.; Maragou, V.I.; Demin, A.K.; Strekalovsky, V.N.; Tsiakaras, P.E. CeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical devices. Int. J. Hydrog. Energy 2011, 36, 6175–6183. [Google Scholar] [CrossRef]
- Stojmenović, M.; Bošković, S.; Žunić, M.; Varela, J.A.; Prekajski, M.; Matović, B.; Mentus, S. Electrical properties of multidoped ceria. Ceram. Int. 2014, 40, 9285–9292. [Google Scholar] [CrossRef]
- Mirković, M.; Dosen, A.; Erić, S.; Stojmenović, M.; Matović, B.; Rosić, A. Structural, Morphological and Electrical Properties of Multi-Doped Calcium Phosphate Materials as Solid Electrolytes for Intermediate Temperature Solid Oxide Fuel Cells. Sci. Sinter. 2018, 50, 95–109. [Google Scholar] [CrossRef]
- Egelja, A.; Pašalić, S.; Dodevski, V.; Kragović, M.; Stojković-Simatović, I.; Radovanović, Ž.; Stojmenović, M. Structural, Morphological and Electrical Properties of Alumina/YAG Composites as Solid Electrolyte for IT—SOFC. Sci. Sinter. 2011, 50, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Sofie, S. Investigation of aluminosilicate as a solid oxide fuel cell refractory. J. Power Sources 2011, 196, 4545–4554. [Google Scholar] [CrossRef]
- Cuia, X.M.; Zhenga, G.J.; Hana, Y.C.; Sua, F.; Zhoub, J. A study on electrical conductivity of chemosynthetic Al2O3–2SiO2 geoploymer materials. J. Power Sources 2008, 184, 652–656. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J.; Separovic, F. 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 2005, 21, 3028–3033. [Google Scholar] [CrossRef]
- Cvetković, V.S.; Purenović, J.M.; Jovićević, J.N. Change of water redox potential, pH and rH in contact with magnesium enriched kaolinite–bentonite ceramics. Appl. Clay Sci. 2008, 38, 268–278. [Google Scholar] [CrossRef]
- Méndez, Y.E.; Vlasova, M.; Leon, I.; Trejo, M.; Kakazey, M. Properties of low-temperature porous ceramics on the base of clay-fusible glass mixtures. J. Aust. Ceram. Soc. 2010, 46, 53–62. [Google Scholar]
- Peng, L.M. Fabrication and mechanical properties of microalloyed and ceramic particulate reinforced NiAl-based alloys. J. Alloys Compd. 2007, 440, 150–153. [Google Scholar] [CrossRef]
- Purenović, J.M. Properties and Application of Multifunctional Microalloyed Composite Alumino-Silicate Ceramics as Active Dielectric with Nanostructured Metal Films on an Amorphous-Crystal Matrix, with the Fractal Nature of Grain Boundary; Monography, Vinča Institut of Nuclear Sciences: Belgrade, Serbia, 2016. [Google Scholar]
- Martínez-González, L.G.; Rodríguez-Reyna, E.; Moreno, K.J.; Escalante-García, J.I.; Fuentes, A.F. Ionic conductivity of apatite-type rare-earth silicates prepared by mechanical milling. J. Alloys Compd. 2009, 476, 710–714. [Google Scholar] [CrossRef]
- Nallamuthu, N.; Prakash, I.; Satyanarayana, N.; Venkateswarlu, M. Electrical conductivity studies of nanocrystalline lanthanum silicate synthesized by sol–gel route. J. Alloys Compd. 2011, 509, 1138–1145. [Google Scholar] [CrossRef]
- Marrero-López, D.; dos Santos-Gómez, L.; León-Reina, L.; Canales-Vázquez, J.; Losilla, E.R. Influence of the microstructure on the bulk and grain boundary conductivity in apatite-type electrolytes. J. Power Sources 2014, 245, 107–118. [Google Scholar] [CrossRef]
- Skinner, S.; KIlner, J. Oxyen ion coductors. Mater. Today 2003, 6, 303–307. [Google Scholar] [CrossRef]
- Slater, P.; Sansom, J.; Tolchard, J. Development of apatite-type oxide ion conductors. Chem. Rec. 2004, 4, 373–384. [Google Scholar] [CrossRef]
- Nenadović, S.; Kljajević, L.J.; Nešić, M.; Petković, M.; Trivunac, K.; Pavlović, V. Structure analysis of geopolymers synthesized from clay originated from Serbia. Environ. Earth Sci. 2017, 2, 76–79. [Google Scholar] [CrossRef]
- Stojmenović, M.; Bošković, S.; Zec, S.; Babić, B.; Matović, B.; Bučevac, D.; Dohčević-Mitrović, Z.; Aldinger, F. Characterization of nanometric multidoped ceria powders. J. Alloys Compd. 2010, 507, 279–285. [Google Scholar] [CrossRef]
- Stojmenović, M.; Bosković, S.; Bučevac, D.; Prekajski, M.; Babić, B.; Matović, B.; Mentus, S. Electrical characterization of multidoped ceria ceramics. Ceram. Int. 2013, 39, 1249–1255. [Google Scholar] [CrossRef]
- Stojmenović, M.; Žunić, M.; Gulicovski, J.; Bajuk–Bogdanović, D.; Holclajtner–Antunović, I.; Dodevski, V.; Menus, S. Structural, morphological, and electrical properties of doped ceria as a solid electrolyte for intermediate–temperature solid oxide fuel cells. J. Mater. Sci. 2015, 50, 3781–3794. [Google Scholar] [CrossRef]
- Tokyo Rigaku Corporation. PDXL Integrated X-ray Powder Diffraction Software; Rigaku Corporation: Tokyo, Japan, 2011. [Google Scholar]
- International Centre for Diffraction Data (ICDD). Powder Doffraction File P-D, Announcement of New Database Release; International Centre for Diffraction Data: Newtown Square, PA, USA, 2012. [Google Scholar]
- Barrett, E.; Joyner, L.; Halenda, P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishii, C.; Ruike, M.; Kuwabara, H. Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon 1992, 30, 1075–1088. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Gadkaree, K.P. Nitrogen adsorption studies of novel synthetic active carbons. J. Colloid Interface Sci. 1997, 192, 250–256. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishii, C.; Kanoh, H.; Hanzawa, Y.; Setoyama, N.; Suzuki, T. Characterization of porous carbons with high resolution αs–analysis and low temperature magnetic susceptibility. Adv. Colloid Interface Sci. 1998, 76–77, 295–320. [Google Scholar] [CrossRef]
- Kljajević, L.Ј.M.; Nenadović, S.S.; Nenadović, M.T.; Bundaleski, N.K.; Todorović, B.Ž.; Pavlović, V.B.; Rakočević, Z.L.J. Structural and chemical properties of thermally treated geopolymer samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Nenadović, S.; Kljajević, L.J.; Nenadović, M.; Mirkovic, M.; Markovic, S. Mechanochemical treatment and structural properties of lead adsorption on kaolinite (Rudovci, Serbia). Environ. Earth Sci. 2015, 73, 7669–7677. [Google Scholar] [CrossRef]
- Ilić, B.; Mitrović, A.; Miličić, L.J. Thermal treatment of kaolin clay. Hem. Ind. 2010, 64, 351–356. [Google Scholar]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T.P. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Revista Mexicana de Fısica 2016, 62, 496–499. [Google Scholar]
- Wang, T.; Sun, D. Preparation and characterization of nanometer-scale powders ceria by electrochemical deposition method. Mater. Res. Bull. 2008, 43, 1745–1760. [Google Scholar] [CrossRef]
- Chelliah, M.; Rayappan, J.; Krishnan, U. Synthesis and Characterization of Cerium Oxide Nanoparticles by Hydroxide Mediated Approach. J. Appl. Sci. 2012, 12, 1734–1737. [Google Scholar]
- Zhang, J.; Ju, X.; Wu, Z.; Liu, T.; Hu, T.D.; Xie, Y.; Zhang, Z. Structural Characteristics of Cerium Oxide Nanocrystals Prepared by the Microemulsion Method. Chem. Mater. 2001, 13, 4192–4197. [Google Scholar] [CrossRef]
- Heah, C.; Khairul Nizar, I.; Kamarudin, H.; Mustafa Al Bakri, A.; Bnhussain, M.; Luqman, M.; Ruzaidi, C.; Liew, Y. Kolin-based geopolymers with various NaOH concentrations. Int. J. Min. Met. Mater. 2013, 20, 313–322. [Google Scholar] [CrossRef]
- Zagaynov, I.; Buryak, A. A surface and catalytic investigation of ceria by laser desorption, ionization mass spectrometry. Nanosyst. Phys. Chem. Math. 2017, 8, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Durmus, S.; Dalmaz, A.; Ozdincer, M.; Sivrikaya, S. Preparation of Cerium Oxide Nanoparticles: An Efficient Catalyst to the Synthesis of Dimeric Disulphide Schiff Bases. CBU J. Sci. 2017, 13, 25–30. [Google Scholar]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages of Fly Ash Geopolymer Gel. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Kumar, S. Synthesis and characterization of geopolymer from Nigerian Clay. Appl. Clay Sci. 2015, 108, 173–181. [Google Scholar] [CrossRef]












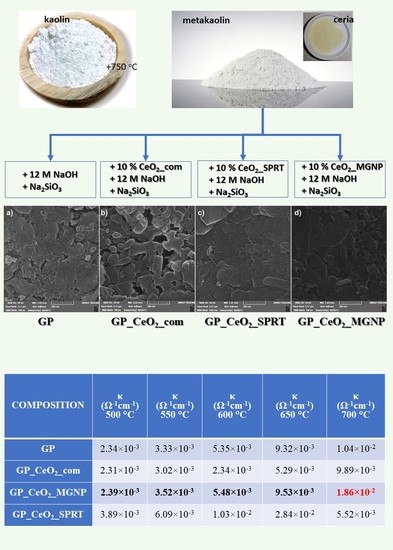
| COMPOSITION | κ (Ω−1 cm−1) 500 °C | κ (Ω−1 cm−1) 550 °C | κ (Ω−1 cm−1) 600 °C | κ (Ω−1 cm−1) 650 °C | κ (Ω−1 cm−1) 700 °C |
|---|---|---|---|---|---|
| GP | 2.34 × 10−3 | 3.33 × 10−3 | 5.35 × 10−3 | 9.32 × 10−3 | 1.04 × 10−2 |
| GP_CeO2_com | 2.31 × 10−3 | 3.02 × 10−3 | 2.34 × 10−3 | 5.29 × 10−3 | 9.89 × 10−3 |
| GP_CeO2_MGNP | 2.39 × 10−3 | 3.52 × 10−3 | 5.48 × 10−3 | 9.53 × 10−3 | 1.86 × 10−2 |
| GP_CeO2_SPRT | 3.89 × 10−3 | 6.09 × 10−3 | 1.03 × 10−2 | 2.84 × 10−2 | 5.52 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulicovski, J.; Nenadović, S.; Kljajević, L.; Mirković, M.; Nišavić, M.; Kragović, M.; Stojmenović, M. Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC. Polymers 2020, 12, 248. https://doi.org/10.3390/polym12010248
Gulicovski J, Nenadović S, Kljajević L, Mirković M, Nišavić M, Kragović M, Stojmenović M. Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC. Polymers. 2020; 12(1):248. https://doi.org/10.3390/polym12010248
Chicago/Turabian StyleGulicovski, Jelena, Snežana Nenadović, Ljiljana Kljajević, Miljana Mirković, Marija Nišavić, Milan Kragović, and Marija Stojmenović. 2020. "Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC" Polymers 12, no. 1: 248. https://doi.org/10.3390/polym12010248
APA StyleGulicovski, J., Nenadović, S., Kljajević, L., Mirković, M., Nišavić, M., Kragović, M., & Stojmenović, M. (2020). Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC. Polymers, 12(1), 248. https://doi.org/10.3390/polym12010248