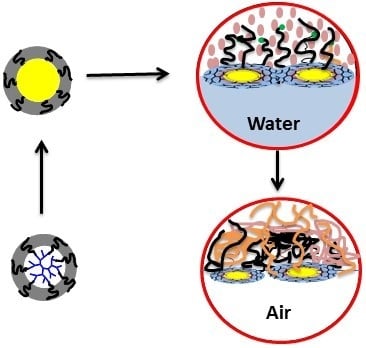
Dendritic Macrosurfactant Assembly for Physical Functionalization of HIPE-Templated Polymers
Abstract
:1. Introduction
2. Synthesis of PEI Macrosurfactants
3. PEI Macrosurfactants as Free Molecular Nanocapsules
4. Direct Dictation of PolyHIPE Surface with PEI Macrosurfactants
5. PEI Macrosurfactant-Aided Metal Nanoparticle-Dictated PolyHIPE
6. PEI Macrosurfactant-Aided AminopolycarboxylicAcid-Dictated PolyHIPE for Metal Ion Adsorption
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.M.; Wang, D.G.; Pan, Q.H.; Gui, Q.Y.; Liao, S.L.; Wang, Y.P. Light-Triggered CO2 Breathing Foam via Nonsurfactant High Internal Phase Emulsion. ACS Appl. Mater. Interfaces 2017, 9, 34497–34505. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Zhang, X.X.; Jin, M.; Wan, D.C. Dendritic Macrosurfactant-Decorated PolyHIPE as a Highly Efficient and Well Recyclable Scavenger of Micropollutants in Water: Topological Effect. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1294–1302. [Google Scholar] [CrossRef]
- Ye, Y.L.; Wan, D.C.; Du, J.; Jin, M.; Pu, H.T. Dendritic Macrosurfactant Mediated Porous Monolith for Eliminating Organic Micropollutants from Water. J. Mater. Chem. A 2015, 3, 6297–6300. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wan, Y.J.; Jin, M.; Wan, D.C. Large-scale preparation of 3D patchy surface with dissimilar dendritic macrosurfactants. Soft Matter 2018, 14, 1043–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.L.; Jin, M.; Wan, D.C. One-Pot to Porous Monolith-Supported Gold Nanoparticles as a Well Recyclable Catalyst. J. Mater. Chem. A 2015, 3, 13519–13525. [Google Scholar] [CrossRef]
- Liu, H.H.; Wan, D.C.; Du, J.; Jin, M. Dendritic Macrosurfactant Mediated One-Pot Preparation of 3D Pt Nanoparticles-Decorated PolyHIPE as a Durable and well Recyclable Catalyst. ACS Appl. Mater. Interfaces 2015, 7, 20885–20892. [Google Scholar] [CrossRef] [PubMed]
- Pulko, I.; Wall, J.; Krajnc, P.; Cameron, N.R. Ultra-High Surface Area Functional Porous Polymers by Emulsion Templating and Hypercrosslinking: Efficient Nucleophilic Catalyst Supports. Chem.-Eur. J. 2010, 16, 2350–2354. [Google Scholar] [CrossRef]
- Wan, Y.J.; Feng, Y.Y.; Wan, D.C.; Jin, M. Polyamino Macrosurfactant Mediated Supporting of Platinum Nanoparticles on PolyHIPE as an over 1500-Time Recyclable Catalyst. RSC Adv. 2016, 6, 109253–109258. [Google Scholar] [CrossRef]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Pashow, K.M.L.; Pribyl, J.G. PolyHIPEs for Separations and Chemical Transformations: A Review. Sol. Extract. Ion Exch. 2019, 37, 1–26. [Google Scholar] [CrossRef]
- Bartl, H.; Bonin, W.V. Uber die Polymerisation in Umgekehrter Emulsion. Makromol. Chem. 1962, 57, 74–95. [Google Scholar] [CrossRef]
- Pulko, I.; Krajnc, P. High Internal Phase Emulsion Templating—A Path to Hierarchically Porous Functional Polymers. Macromol. Rapid Commun. 2012, 33, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320. [Google Scholar] [CrossRef]
- Wu, D.C.; Xu, F.; Sun, B.; Fu, R.W.; He, H.K.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.; Ungureanu, S.; Deleuze, H.; Backov, R. Hybrid Foams, Colloids and beyond: From Design to Applications. Chem. Soc. Rev. 2011, 40, 771–788. [Google Scholar] [CrossRef]
- Kimmins, S.D.; Cameron, N.R. Functional Porous Polymers by Emulsion Templating: Recent Advances. Adv. Funct. Mater. 2011, 21, 211–225. [Google Scholar] [CrossRef]
- Kovacic, S.; Preishuber-Pflugl, F.; Pahovnik, D.; Zagar, E.; Slugovc, C. Covalent incorporation of the surfactant into high internal phase emulsion templated polymeric foams. Chem. Commun. 2015, 51, 7725–7728. [Google Scholar] [CrossRef]
- Zhang, T.; Silverstein, M.S. Microphase-Separated Macroporous Polymers from an Emulsion-Templated Reactive Triblock Copolymer. Macromolecules 2018, 51, 3828–3835. [Google Scholar] [CrossRef]
- Bancroft, W.D. The theory of emulsification V. J. Phys. Chem. 1913, 17, 501–519. [Google Scholar] [CrossRef] [Green Version]
- van Hest, J.C.M.; Delnoye, D.A.P.; Baars, M.W.P.L.; van Genderen, M.H.P.; Meijer, E.W. Polystyrene-dendrimer amphiphilic block-copolymers with a generation-dependent aggregation. Science 1995, 268, 1592–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.F.; Yan, D.Y. Supramolecular self-assembly of giant polymer vesicles with controlled sizes. Angew. Chem. Int. Ed. 2004, 43, 4896–4899. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-Mediated Self-Assembly, Disassembly, and Self-Organization of Complex Systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.F.; Zhou, Y.F.; Yan, D.Y. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef]
- Kramer, M.; Stumbe, J.F.; Turk, H.; Krause, S.; Komp, A.; Delineau, L.; Prokhorova, S.; Kautz, H.; Haag, R. pH-responsive molecular nanocarriers based on dendritic core-shell architectures. Angew. Chem. Int. Ed. 2002, 41, 4252–4256. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Pastor-Perez, L.; Frey, H.; Stiriba, S.E. Role of topology and amphiphilicity for guest encapsulation in functionalized hyperbranched poly(ethylenimine)s. Macromolecules 2005, 38, 227–229. [Google Scholar] [CrossRef]
- Wan, D.C.; Lai, Y.; Jin, M.; Pu, H.T. Selective Encapsulation of Ionic Dyes by Core/Shell Amphiphilic Macromolecules Derived from Hyperbranched Polyethylenimine:Properties through Structures. Macromol. Chem. Phys. 2011, 212, 1910–1917. [Google Scholar]
- Wan, D.C.; Jin, M.; Pu, H.T. Charge-Selective Separation and Recovery of Organic Ions by Polymeric Micelles. J. Polym. Sci. Part B Polym Phys. 2014, 52, 872–881. [Google Scholar] [CrossRef]
- Antonietti, L.; Aymonier, C.; Schlotterbeck, U.; Garamus, V.M.; Maksimova, T.; Richtering, W.; Mecking, S. Core-shell-structured highly branched poly(ethylenimine amide)s: Synthesis and structure. Macromolecules 2005, 38, 5914–5920. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.J.; Chen, Y.; Zhu, D.D.; Shen, Z.; Stiriba, S.E. Hyperbranched polyethylenimines as versatile precursors for the preparation of different type of unimolecular micelles. React. Funct. Polym. 2007, 67, 383–395. [Google Scholar] [CrossRef]
- Cao, X.L.; Li, Z.Q.; Song, X.W.; Cui, X.H.; Cao, P.F.; Liu, H.J.; Cheng, F.; Chen, Y. Core-shell type multiarm star poly(epsilon-caprolactone) with high molecular weight hyperbranched polyethylenimine as core: Synthesis, characterization and encapsulation properties. Eur. Polym. J. 2008, 44, 1060–1070. [Google Scholar] [CrossRef]
- Wan, D.C.; Pu, H.T.; Cai, X.Y. Separation Promoted by Molecular Recognition of a Core Engineered Macromolecular Nanocapsule. Macromolecules 2008, 41, 7787–7789. [Google Scholar] [CrossRef]
- Wan, D.C.; Wang, G.C.; Pu, H.T.; Jin, M. Can Nonspecific Host-Guest Interaction Lead to Highly Specific Encaspulation by a Supramolecular Nanocapsule? Macromolecules 2009, 42, 6448–6456. [Google Scholar] [CrossRef]
- Wan, D.C.; Yuan, J.J.; Pu, H.T. Macromolecular Nancapsule Derived from Hyperbranched Polyethylenimine (HPEI): Mechanism of Guest Encapsulation versus Molecular Parameters. Macromolecules 2009, 42, 1533–1540. [Google Scholar] [CrossRef]
- Wan, D.C.; Pu, H.T.; Jin, M. Highly Specific Molecular Recognition by a Roughly-Defined Supramolecular Nanocapsule: A Fuzzy Recognition Mechanism. Macromolecules 2010, 43, 3809–3816. [Google Scholar] [CrossRef]
- Wan, D.C.; Pu, H.T.; Jin, M.; Wang, G.W.; Huang, J.L. Supramolecular Fuzzy Recognition Leads to Effective Differentiation of Similar Molecules. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2373–2381. [Google Scholar] [CrossRef]
- Wan, D.C.; Jin, M.; Pu, H.T.; Pan, H.Y.; Chang, Z.H. Enhancing the Unimolecularity and Control for Guest Release of a Macromolecular Nanocapsule via Core Engineering. React. Funct. Polym. 2010, 70, 916–922. [Google Scholar] [CrossRef]
- Viswanathan, P.; Johnson, D.W.; Hurley, C.; Cameron, N.R.; Battaglia, G. 3D Surface Functionalization of Emulsion-Templated Polymeric Foams. Macromolecules 2014, 47, 7091–7098. [Google Scholar] [CrossRef]
- Gui, H.G.; Guan, G.W.; Zhang, T.; Guo, Q.P. Microphase-separated, hierarchical macroporous polyurethane from a nonaqueous emulsion-templated reactive block copolymer. Chem. Eng. J. 2019, 365, 369–377. [Google Scholar] [CrossRef]
- Zhang, T.; Silverstein, M.S. Robust, highly porous hydrogels templated within emulsions stabilized using a reactive, crosslinking triblock copolymer. Polymer 2019, 168, 146–154. [Google Scholar] [CrossRef]
- Mathieu, K.; Jerome, C.; Debuigne, A. Influence of the Macromolecular Surfactant Features and Reactivity on Morphology and Surface Properties of Emulsion-Templated Porous Polymers. Macromolecules 2015, 48, 6489–6498. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, Y.; Lamson, M.; Silverstein, M.S.; Tilton, R.D.; Matyjaszewski, K. PEO-Based Star Copolymers as Stabilizers for Water-in-Oil or Oil-in-Water Emulsions. Macromolecules 2012, 45, 9419–9426. [Google Scholar] [CrossRef]
- Xie, G.J.; Krys, P.; Tilton, R.D.; Matyjaszewski, K. Heterografted Molecular Brushes as Stabilizers for Water-in-Oil Emulsions. Macromolecules 2017, 50, 2942–2950. [Google Scholar] [CrossRef]
- Xu, Z.S.; Ford, W.T. Polystyrene latexes containing poly(propyleneimine) dendrimers. Macromolecules 2002, 35, 7662–7668. [Google Scholar] [CrossRef]
- Yi, C.F.; Shen, Y.H.; Deng, Z.W.; Xu, Z.S.; Ford, W.T. Emulsion polymerization of styrene containing dendrimer PAMAM. Acta Polym. Sin. 2004, 6, 831–834. [Google Scholar]
- Christian, D.A.; Tian, A.W.; Ellenbroek, W.G.; Levental, I.; Rajagopal, K.; Janmey, P.A.; Liu, A.J.; Baumgart, T.; Discher, D.E. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nat. Mater. 2009, 8, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.R.; Sherrington, D.C.; Albiston, L.; Gregory, D.P. Study of the formation of the open cellular morphology of poly(styrene/divinylbenzene) polyHIPE materials by cryo-SEM. Colloid Polym. Sci. 1996, 274, 592–595. [Google Scholar] [CrossRef]
- Menner, A.; Bismarck, A. New evidence for the mechanism of the pore formation in polymerising High Internal Phase Emulsions or why polyHIPEs have an interconnected pore network structure. Macromol. Symp. 2006, 242, 19–24. [Google Scholar] [CrossRef]
- Li, C.H.; Jin, M.; Wan, D.C. Evolution of a Radical-Triggered Polymerizing High Internal Phase Emulsion into an Open-Cellular Monolith. Macromol. Chem. Phys. 2019, 220, 1900216. [Google Scholar] [CrossRef]
- Ikem, V.O.; Menner, A.; Horozov, T.S.; Bismarck, A. Highly Permeable Macroporous Polymers Synthesized from Pickering Medium and High Internal Phase Emulsion Templates. Adv. Mater. 2010, 22, 3588–3592. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.X.; Zhu, Y.; Zhou, C.; Zhang, S.M. Pickering emulsion-templated polymers: Insights into the relationship between surfactant and interconnecting pores. RSC Adv. 2019, 9, 18909–18916. [Google Scholar] [CrossRef] [Green Version]
- Tebboth, M.; Kogelbauer, A.; Bismarck, A. Liquid-Liquid Extraction within Emulsion Templated Macroporous Polymers. Ind. Eng. Chem. Res. 2015, 54, 5974–5981. [Google Scholar] [CrossRef]
- Kovacic, S.; Anzlovar, A.; Erjavec, B.; Kapun, G.; Matsko, N.B.; Zigon, M.; Zagar, E.; Pintar, A.; Slugovc, C. Macroporous ZnO Foams by High Internal Phase Emulsion Technique: Synthesis and Catalytic Activity. ACS Appl. Mater. Interfaces 2014, 6, 19075–19081. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, S.M.; Zhu, Y.; Chu, Y.Q.; Chen, J.D. Hydrophilic polymer foams with well-defined open-cell structure prepared from Pickering high internal phase emulsions. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2181–2187. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, S.M.; Chen, J.D.; Zhu, Y. Switchable release and recovery of nanoparticles via a Pickering-emulsion-templated porous carrier. J. Mater. Chem. A 2013, 1, 13970–13977. [Google Scholar] [CrossRef]
- Repo, E.; Warch, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpaa, M. Aminopolycarboxylic Acid Functionalized Adsorbents for Heavy Metals Removal from Water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef]
- Weng, S.Q.; Jin, M.; Wan, D.C. Macrosurfactant Mediated, Aminopolycarboxy Acid Dictated and Open-cellular Adsorbent for Removing Metal Micropollutants from Water. Mater. Chem. Front. 2020, 4, 985–995. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Weng, S.; Jin, M.; Wan, D. Dendritic Macrosurfactant Assembly for Physical Functionalization of HIPE-Templated Polymers. Polymers 2020, 12, 779. https://doi.org/10.3390/polym12040779
Li C, Weng S, Jin M, Wan D. Dendritic Macrosurfactant Assembly for Physical Functionalization of HIPE-Templated Polymers. Polymers. 2020; 12(4):779. https://doi.org/10.3390/polym12040779
Chicago/Turabian StyleLi, Chenhui, Shiqi Weng, Ming Jin, and Decheng Wan. 2020. "Dendritic Macrosurfactant Assembly for Physical Functionalization of HIPE-Templated Polymers" Polymers 12, no. 4: 779. https://doi.org/10.3390/polym12040779
APA StyleLi, C., Weng, S., Jin, M., & Wan, D. (2020). Dendritic Macrosurfactant Assembly for Physical Functionalization of HIPE-Templated Polymers. Polymers, 12(4), 779. https://doi.org/10.3390/polym12040779