Influence of Different Types of Peroxides on the Long-Chain Branching of PP via Reactive Extrusion
Abstract
:1. Introduction
- Increasing the average molecular weight of PP
- Broadening the molecular weight distribution by incorporating high and low molecular weight chain fractions
- Blending PP with polymers such as PE-LD
- Introducing long-chain branching (LCB) on the backbone of PP, which is the most popular method to obtain PP-HMS
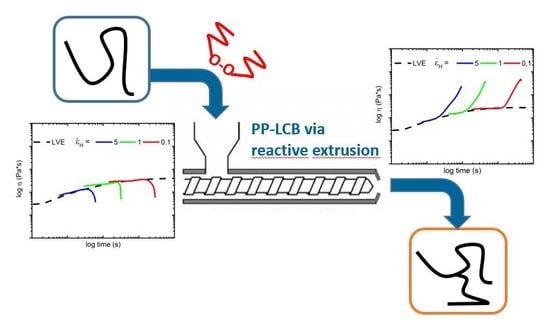
- PP-LCB can be achieved in different ways, but reactive extrusion is the most popular process. This type of process offers very short reaction times, little or no use of solvents, simple product isolation steps, and relatively low infrastructure costs [11,12]. In the literature, PP-LCB is a well-studied topic with different approaches to improve the properties of linear PP and their disadvantages [13,14,15,16,17,18,19,20,21,22]. In addition to introducing LCB onto the backbone of PP, further competition reactions can take place. PP in particular tends to undergo chain scission reactions, so-called β-scission, which creates smaller unsaturated fragments of chains [23,24]. There is a possibility that cross-linking occurs, but a prediction of this phenomenon is very difficult. The phenomenon of β-scission caused by the use of peroxides is also known as controlled rheology PP (PP-CR). This leads to a reduction in viscosity, which results in a reduction in average molar mass and a narrowing of PP’s molar mass distribution (MMD) [25]. Additional side reactions could occur, such as disproportionation or a combination of free peroxide radicals. In particular, at higher temperatures, the equilibrium shifts to β-scission or disproportionation, which negatively affects the branching [24]. For this reason, the extrusion temperatures in scientific works are mainly at lower temperatures (180 °C). However, in practice, high temperatures have been used for the extrusion process, which means that additional effects may have occurred such as increased chain scission or different reaction rates during the extrusion. Figure 1 shows a schematic representation of the LCB reaction process confronted with the β-scission of PP. In the first step, the peroxide decomposes under homolytic scission into two primary radicals, which abstract a hydrogen atom from the PP backbone and therefore a PP macroradical is generated. There are now two possible reactions that can take place—on the one hand, the LCB, and on the other hand, the β-scission of PP [26,27].
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization of Molar Mass and Viscosity
2.4. Mechanical Testing—Tensile Test and Tensile Impact Strength
3. Results and Discussion
3.1. Melt Flow Properties
3.2. Dynamic Rheology
3.3. Molar Mass Determination
3.4. Extensional Rheology
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts 2019; Plastics Europe—Association of Plastics Manufacturers: Brussels, Belgium, 2019.
- Pasquini, N.; Addeo, A. Polypropylene Handbook; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Jahani, Y.; Ghetmiri, M.; Vaseghi, M.R. The effects of long chain branching of polypropylene and chain extension of poly(ethylene terephthalate) on the thermal behavior, rheology and morphology of their blends. RSC Adv. 2015, 5, 21620–21628. [Google Scholar] [CrossRef]
- Borsig, E.; Fiedlerová, A.; Rychlá, L.; Lazár, M.; Rätzsch, M.; Haudel, G. Crosslinking of polypropylene–polyethylene blends by peroxide and the effect of pentaerythritol tetrallyl ether. J. Appl. Polym. Sci. 1989, 37, 467–478. [Google Scholar] [CrossRef]
- Graebling, D. Synthesis of Branched Polypropylene by a Reactive Extrusion Process. Macromolecules 2002, 35, 4602–4610. [Google Scholar] [CrossRef]
- Augier, S.; Coiai, S.; Passaglia, E.; Ciardelli, F.; Zulli, F.; Andreozzi, L.; Giordano, M. Structure and rheology of polypropylene with various architectures prepared by coagent-assisted radical processing. Polym. Int. 2010, 59, 1499–1505. [Google Scholar] [CrossRef]
- Guo, P.; Xu, Y.; Lu, M.; Zhang, S. High Melt Strength Polypropylene with Wide Molecular Weight Distribution Used as Basic Resin for Expanded Polypropylene Beads. Ind. Eng. Chem. Res. 2015, 54, 217–225. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Wu, F.; Pang, Y.; Liu, W.; Zhai, W.; Zheng, W. A new strategy for preparation of long-chain branched polypropylene via reactive extrusion with supercritical CO2 designed for an improved foaming approach. J. Mater. Sci. 2016, 51, 2705–2715. [Google Scholar] [CrossRef]
- Chikhalikar, K.; Banik, S.; Azad, L.B.; Jadhav, K.; Mahajan, S.; Ahmad, Z.; Kulkarni, S.; Gupta, S.; Doshi, P.; Pol, H.; et al. Extrusion film casting of long chain branched polypropylene. Polym. Eng. Sci. 2015, 55, 1977–1987. [Google Scholar] [CrossRef]
- Moad, G. The synthesis of polyolefin graft copolymers by reactive extrusion. Prog. Polym. Sci. 1999, 24, 81–142. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Kruliš, Z.; Kokta, B.V.; Horák, Z.; Michálková, D.; Fortelný, I. Compatibilization as a Procedure for Recycling of Commingled Polyolefin Waste. Macromol. Mater. Eng. 2001, 286, 156–160. [Google Scholar] [CrossRef]
- Hettema, R.; Pasman, J.; Janssen, L.P.B.M. Reactive extrusion of recycled bottle waste material. Polym. Eng. Sci. 2002, 42, 665–680. [Google Scholar] [CrossRef] [Green Version]
- Parent, J.S.; Sengupta, S.S.; Kaufman, M.; Chaudhary, B.I. Coagent-induced transformations of polypropylene microstructure: Evolution of bimodal architectures and cross-linked nano-particles. Polymer 2008, 49, 3884–3891. [Google Scholar] [CrossRef]
- Parent, J.S.; Bodsworth, A.; Sengupta, S.S.; Kontopoulou, M.; Chaudhary, B.I.; Poche, D.; Cousteaux, S. Structure–rheology relationships of long-chain branched polypropylene: Comparative analysis of acrylic and allylic coagent chemistry. Polymer 2009, 50, 85–94. [Google Scholar] [CrossRef]
- El Mabrouk, K.; Parent, J.S.; Chaudhary, B.I.; Cong, R. Chemical modification of PP architecture: Strategies for introducing long-chain branching. Polymer 2009, 50, 5390–5397. [Google Scholar] [CrossRef]
- Vergnes, B.; Berzin, F. Modeling of reactive systems in twin-screw extrusion: Challenges and applications. Comptes Rendus Chim. 2006, 9, 1409–1418. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, G.K.; Kommoji, S.; Banerjee, R.; Ghosh, A.K. The effect of material characteristics and mould parameters on the thermoforming of thick polypropylene sheets. J. Plast. Film Sheeting 2014, 30, 162–180. [Google Scholar] [CrossRef]
- Passaglia, E.; Coiai, S.; Cicogna, F.; Ciardelli, F. Some recent advances in polyolefin functionalization. Polym. Int. 2014, 63, 12–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, F.; Zhang, H. Isothermal and Non-Isothermal Crystallization Studies of Long Chain Branched Polypropylene Containing Poly(ethylene-co-octene) under Quiescent and Shear Conditions. Polymers 2017, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Cha, J.H.; Seo, K.H. Effects of chain extender on properties and foaming behavior of polypropylene foam. RSC Adv. 2019, 9, 25496–25507. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.P. Polypropylene Handbook: Polymerization, Characterization, Properties, Processing, Applications; Hanser Publisher: New York, NY, USA, 1996. [Google Scholar]
- Maier, R.-D.; Schiller, M. Handbuch Kunststoff Additive; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2016. [Google Scholar]
- Azizi, H.; Ghasemi, I. Reactive extrusion of polypropylene: Production of controlled-rheology polypropylene (CRPP) by peroxide-promoted degradation. Polym. Test. 2004, 23, 137–143. [Google Scholar] [CrossRef]
- Su, F.-H.; Huang, H.-X. Rheology and melt strength of long chain branching polypropylene prepared by reactive extrusion with various peroxides. Polym. Eng. Sci. 2010, 50, 342–351. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, J.; Li, L.; Zhao, S.; Shi, Y.; Xin, Z. Relationship between Peroxide Initiators and Properties of Styrene Grafted Polypropylene via Reactive Extrusion. J. Macromol. Sci. Part B 2018, 57, 377–394. [Google Scholar] [CrossRef]
- Lagendijk, R.P.; Hogt, A.H.; Buijtenhuijs, A.; Gotsis, A.D. Peroxydicarbonate modification of polypropylene and extensional flow properties. Polymer 2001, 42, 10035–10043. [Google Scholar] [CrossRef]
- Gotsis, A.D.; Zeevenhoven, B.L.F.; Hogt, A.H. The effect of long chain branching on the processability of polypropylene in thermoforming. Polym. Eng. Sci. 2004, 44, 973–982. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.M. Upcycling of polypropylene—The influence of polyethylene impurities. Polym. Eng. Sci. 2017, 57, 1374–1381. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Archodoulaki, V.-M. Long chain branching as an innovative up-cycling process of polypropylene post-consumer waste—Possibilities and limitations. Waste Manag. 2017, 68, 32–37. [Google Scholar] [CrossRef]
- Kamleitner, F.; Duscher, B.; Koch, T.; Knaus, S.; Schmid, K.; Archodoulaki, V.-M. Influence of the Molar Mass on Long-Chain Branching of Polypropylene. Polymers 2017, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Borsig, E.; Fiedlerová, A.; Lazár, M. Efficiency of Chemical Cross-Linking of Polypropylene. J. Macromol. Sci. Part A Chem. 1981, 16, 513–528. [Google Scholar] [CrossRef]
- Chodák, I.; Lazár, M. Effect of the type of radical initiator on crosslinking of polypropylene. Die Angew. Makromol. Chem. 1982, 106, 153–160. [Google Scholar] [CrossRef]
- Klenk, H.; Götz, P.H.; Siegmeier, R.; Mayr, W. Peroxy Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Takamura, M.; Nakamura, T.; Takahashi, T.; Koyama, K. Effect of type of peroxide on cross-linking of poly(l-lactide). Polym. Degrad. Stab. 2008, 93, 1909–1916. [Google Scholar] [CrossRef]
- ŞIRIN, K.; Yavuz, M.; Canli, M.; Doğan, F. Improving the Mechanical, Physical, Thermal, and Morphological Properties of Isotactic Polypropylene with Dialkylperoxide. J. Polym. Mater. 2013, 30, 383–396. [Google Scholar]
- Yavuz, M.; Çanli, M. Influence of Dilauroyl Peroxide on Mechanical and Thermal Properties of Different Polypropylene Matrices. Polym. Korea 2015, 39, 200–209. [Google Scholar]
- Yin, F.; Chen, Q.; Lin, J.; Deng, Y.; Mao, X. Effect of different peroxide initiators on the reaction extrusion of polypropylene-graft-cardanol and its compatibilization on PP/PC. J. Polym. Res. 2014, 21, 411. [Google Scholar] [CrossRef]
- Giles, H.F., Jr.; Mount, E.M., III; Wagner, J.R., Jr. Extrusion: The Definitive Processing Guide and Handbook; William Andrew: Park Ridge, NJ, USA, 2004. [Google Scholar]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Vincentz Network GmbH & Co KG: Hannover, Germany, 2006. [Google Scholar]
- Vlachopoulos, J.; Strutt, D. The role of rheology in polymer extrusion. In Proceedings of the New Technology for Extrusion Conference, Milan, Italy, 20–21 November 2003; pp. 20–21. [Google Scholar]
- An, Y.; Zhang, Z.; Bi, W.; Wang, Y.; Tang, T. Characterization of high melt strength polypropylene synthesized via silane grafting initiated by in situ heat induction reaction. J. Appl. Polym. Sci. 2008, 110, 3727–3732. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Wang, L.; Wan, D.; Zhang, Z.; Liu, F.; Xing, H.; Wang, Y.; Tang, T. Synthesis and Structure–Property Relationships of Polypropylene-g-poly(ethylene-co-1-butene) Graft Copolymers with Well-Defined Long Chain Branched Molecular Structures. Macromolecules 2011, 44, 4167–4179. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Liao, X.; Yang, Q. The molecular structure characteristics of long chain branched polypropylene and its effects on non-isothermal crystallization and mechanical properties. Polymer 2013, 54, 1455–1462. [Google Scholar] [CrossRef]
- Cao, J.; Wen, N.; Zheng, Y.-Y. Effect of long chain branching on the rheological behavior, crystallization and mechanical properties of polypropylene random copolymer. Chin. J. Polym. Sci. 2016, 34, 1158–1171. [Google Scholar] [CrossRef]
- Li, Y.; Jia, S.; Du, S.; Wang, Y.; Lv, L.; Zhang, J. Improved properties of recycled polypropylene by introducing the long chain branched structure through reactive extrusion. Waste Manag. 2018, 76, 172–179. [Google Scholar] [CrossRef]
- Malmberg, A.; Gabriel, C.; Steffl, T.; Münstedt, H.; Löfgren, B. Long-Chain Branching in Metallocene-Catalyzed Polyethylenes Investigated by Low Oscillatory Shear and Uniaxial Extensional Rheometry. Macromolecules 2002, 35, 1038–1048. [Google Scholar] [CrossRef]
- Bernreitner, K.; Neiβl, W.; Gahleitner, M. Correlation between molecular structure and rheological behaviour of polypropylene. Polym. Test. 1992, 11, 89–100. [Google Scholar] [CrossRef]
- Morshedian, J.; Karbalaei-Bagher, M.; Bayazian, H.; Jamshidi, A.; Razavi-Nouri, M. Unraveling the Bimodality of Polypropylene Film Grades Using Rheological Shear and Elongational Measurements: Inconsistent Results of Gel Permeation Chromatography. Polym. Sci. Ser. A 2018, 60, 523–529. [Google Scholar] [CrossRef]
- Fleissner, M. Characterization of polymer molecular mass distribution from rheological measurements. Makromol. Chem. Macromol. Symp. 1992, 61, 324–341. [Google Scholar] [CrossRef]
- Tzoganakis, C. A rheological evaluation of linear and branched controlled-rheology polypropylenes. Can. J. Chem. Eng. 1994, 72, 749–754. [Google Scholar] [CrossRef]
- Vaezi, J.; Nekoomanesh, M.; Khonakdar, H.A.; Jafari, S.H.; Komber, H.; Mojarrad, A. On the Correlation of Rheology and Morphology of Bimodal Polypropylene Reactor Blends Synthesized by Homogeneous Binary Metallocene/Metallocene Catalysts. Polym. Plast. Technol. Eng. 2018, 57, 791–803. [Google Scholar] [CrossRef]
- Mezger, T.G. Das Rheologie Handbuch: 5; FARBE UND LACK; Vincentz Network GmbH & Co KG: Hannover, Germany, 2016. [Google Scholar]
- Wood-Adams, P.M.; Dealy, J.M.; deGroot, A.W.; Redwine, O.D. Effect of Molecular Structure on the Linear Viscoelastic Behavior of Polyethylene. Macromolecules 2000, 33, 7489–7499. [Google Scholar] [CrossRef]
- DeMaio, V.; Dong, D. The effect of chain structure on melt strength of polypropylene and polyethylene. In Technical Papers of the Annual Technical Conference; Society of Plastics Engineers Incorporated: Brookfield, CT, USA, 1997; pp. 1512–1516. [Google Scholar]
- Sugimoto, M.; Masubuchi, Y.; Takimoto, J.; Koyama, K. Melt rheology of polypropylene containing small amounts of high molecular weight chain. I. Shear flow. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2692–2704. [Google Scholar] [CrossRef]
- Watanabe, H.; Kotaka, T. Viscoelastic properties and relaxation mechanisms of binary blends of narrow molecular weight distribution polystyrenes. Macromolecules 1984, 17, 2316–2325. [Google Scholar] [CrossRef]
- Watanabe, H.; Sakamoto, T.; Kotaka, T. Viscoelastic properties of binary blends of narrow molecular weight distribution polystyrenes. 2. Macromolecules 1985, 18, 1008–1015. [Google Scholar] [CrossRef]
- Masuda, T.; Kitagawa, K.; Inoue, T.; Onogi, S. Rheological Properties of Anionic Polystyrenes. II. Dynamic Viscoelasticity of Blends of Narrow-Distribution Polystyrenes. Macromolecules 1970, 3, 116–125. [Google Scholar] [CrossRef]
- Graessley, W.W. Some phenomenological consequences of the Doi–Edwards theory of viscoelasticity. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 27–34. [Google Scholar] [CrossRef]
- Dealy, J.M.; Read, D.J.; Larson, R.G. Structure and Rheology of Molten Polymers: From Structure to Flow Behavior and Back Again; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2018. [Google Scholar]
- Guapacha, J.; Vallés, E.M.; Quinzani, L.M.; Failla, M.D. Long-chain branched polypropylene obtained using an epoxy resin as crosslinking agent. Polym. Bull. 2017, 74, 2297–2318. [Google Scholar] [CrossRef]
- Guapacha, J.; Vallés, E.M.; Failla, M.D.; Quinzani, L.M. Efficiency of Different Chain-Linking Agents in the Synthesis of Long-Chain Branched Polypropylene: Molecular, Thermal, and Rheological Characterization. Polym. Plast. Technol. Eng. 2018, 57, 1209–1224. [Google Scholar] [CrossRef]
- Chikhalikar, K.; Deshpande, A.; Pol, H.; Dhoble, D.; Jha, S.; Jadhav, K.; Mahajan, S.; Ahmad, Z.; Kulkarni, S.; Gupta, S.; et al. Long chain branched impact copolymer of polypropylene: Microstructure and rheology. Polym. Eng. Sci. 2015, 55, 1463–1474. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Wu, C. The Effect of PP and Peroxide on the Properties and Morphology of HDPE and HDPE/PP Blends. Adv. Polym. Technol. 2013, 32. [Google Scholar] [CrossRef]
- Drabek, J.; Zatloukal, M.; Martyn, M. Effect of molecular weight, branching and temperature on dynamics of polypropylene melts at very high shear rates. Polymer 2018, 144, 179–183. [Google Scholar] [CrossRef]
- Münstedt, H. Extensional Rheology and Processing of Polymeric Materials. Int. Polym. Proc. 2018, 33, 594–618. [Google Scholar] [CrossRef]
- Duscher, B.; Schausberger, A. Influence of processing on the flow properties of long-chain branched polypropylene. Polym. Eng. Sci. 2018, 58, 1596–1603. [Google Scholar] [CrossRef]
- Macosko, C.W.; Larson, R.G. Rheology: Principles, Measurements, and Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: Berlin/Heidelberg, Germany, 2007; Volume 1076. [Google Scholar]
- Scheirs, J. Compositional and Failure Analysis of Polymers: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Lampman, S. Characterization and Failure Analysis of Plastics; Asm International: Cleveland, OH, USA, 2003. [Google Scholar]
- Grellmann, W.; Seidler, S. Kunststoffprüfung; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2015. [Google Scholar]
- Moser, A.; Feuchter, M.J.P. Mechanical properties of composites used in high-voltage applications. Polymers 2016, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Ibhadon, A.O. Fracture mechanics of polypropylene: Effect of molecular characteristics, crystallization conditions, and annealing on morphology and impact performance. J. Appl. Polym. Sci. 1998, 69, 2657–2661. [Google Scholar] [CrossRef]
- Tzoganakis, C.; Vlachopoulos, J.; Hamielec, A.E.; Shinozaki, D.M. Effect of molecular weight distribution on the rheological and mechanical properties of polypropylene. Polym. Eng. Sci. 1989, 29, 390–396. [Google Scholar] [CrossRef]













| Peroxide | CAS-No.: | Max. Storage Temperature (°C) | Molecular Weight (g/mol) | Active Oxygen Assay (%) | 10 h Half-Life Temperature (°C) |
|---|---|---|---|---|---|
| PODIC C126 * | 53220-22-7 | 15 | 514.8 | 2.95 | 48 |
| LP * | 105-74-8 | 30 | 398.6 | 3.97 | 61 |
| BIC ** | 2372-21-6 | 25 | 176.2 | 6.81 | 98 |
| BEC ** | 34443-12-4 | 30 | 246.3 | 6.17 | 98 |
| AEC ** | 70833-40-8 | 25 | 260.4 | 5.78 | 95 |
| Sample | Extrusion Temperature (°C) | Sample Specification |
|---|---|---|
| PP 180/PP 240 | 180/240 | Virgin PP granulate |
| PODIC 180/PODIC 240 | 180/240 | 20 mmol/kg PODIC C126 (1 wt %) |
| BIC 180/BIC 240 | 180/240 | 20 mmol/kg BIC (0.5 wt %) |
| BEC 180/BEC 240 | 180/240 | 20 mmol/kg BEC (0.5 wt %) |
| AEC 180/AEC 240 | 180/240 | 20 mmol/kg AEC (0.5 wt %) |
| LP 180/LP 240 | 180/240 | 20 mmol/kg LP (0.8 wt %) |
| Sample | ωC (rad/s) | GC (kPa) | Comment |
|---|---|---|---|
| PP 180 | 36 | 23.9 | |
| PODIC 180 | 50 | 24.5 | MW ↓, MMD ↓ |
| BIC 180 | 139 | 29.0 | MW ↓, MMD ↓ |
| BEC 180 | - | - | (MW ↓, MMD ↓) * |
| AEC 180 | 460 | 33.6 | MW ↓, MMD ↓ |
| LP 180 | 84 | 25.6 | MW ↓, MMD ↓ |
| PP 240 | 98 | 27.1 | |
| PODIC 240 | 66 | 23.5 | MW ↑, MMD ↑ |
| BIC 240 | 308 | 32.6 | MW ↓, MMD ↓ |
| BEC 240 | - | - | (MW ↓, MMD ↓) * |
| AEC 240 | - | - | (MW ↓, MMD ↓) * |
| LP 240 | 114 | 28.4 | MW ↓, MMD ↓ |
| Sample | Mw (kg mol−1) | Mn (kg mol−1) | Mw/Mn |
|---|---|---|---|
| PP 180 | 379 | 63.2 | 6.0 |
| PODIC 180 | 359 | 62.8 | 5.7 |
| BIC 180 | 263 | 57.4 | 4.6 |
| BEC 180 | 164 | 48.3 | 3.4 |
| AEC 180 | 179 | 50.1 | 3.6 |
| LP 180 | 307 | 59.9 | 5.1 |
| PP 240 | 354 | 62.2 | 5.7 |
| PODIC 240 | 309 | 60.5 | 5.1 |
| BIC 240 | 230 | 54.1 | 4.2 |
| BEC 240 | 160 | 46.8 | 3.4 |
| AEC 240 | 179 | 50.0 | 3.6 |
| LP 240 | 341 | 62.0 | 5.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanic, S.; Gottlieb, G.; Koch, T.; Göpperl, L.; Schmid, K.; Knaus, S.; Archodoulaki, V.-M. Influence of Different Types of Peroxides on the Long-Chain Branching of PP via Reactive Extrusion. Polymers 2020, 12, 886. https://doi.org/10.3390/polym12040886
Stanic S, Gottlieb G, Koch T, Göpperl L, Schmid K, Knaus S, Archodoulaki V-M. Influence of Different Types of Peroxides on the Long-Chain Branching of PP via Reactive Extrusion. Polymers. 2020; 12(4):886. https://doi.org/10.3390/polym12040886
Chicago/Turabian StyleStanic, Sascha, Gergö Gottlieb, Thomas Koch, Lukas Göpperl, Klaus Schmid, Simone Knaus, and Vasiliki-Maria Archodoulaki. 2020. "Influence of Different Types of Peroxides on the Long-Chain Branching of PP via Reactive Extrusion" Polymers 12, no. 4: 886. https://doi.org/10.3390/polym12040886
APA StyleStanic, S., Gottlieb, G., Koch, T., Göpperl, L., Schmid, K., Knaus, S., & Archodoulaki, V. -M. (2020). Influence of Different Types of Peroxides on the Long-Chain Branching of PP via Reactive Extrusion. Polymers, 12(4), 886. https://doi.org/10.3390/polym12040886