Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction
Abstract
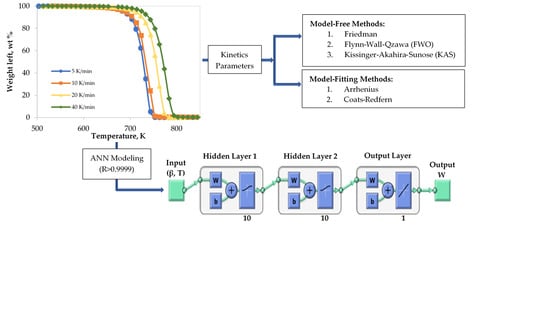
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Decomposition of LDPE
2.3. Kinetic Theory
2.4. Topology of ANNs
3. Results and Discussion
3.1. TG-DTG Analysis of LDPE
3.2. Model-Free Kinetics Calculation
3.3. Model-Fitting Kinetics Calculation
3.4. Pyrolysis Prediction by ANN Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miskolczi, N.; Bartha, L.; Deák, G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feed stocks. Polym. Degrad. Stabil. 2006, 91, 517–526. [Google Scholar] [CrossRef]
- Diaz Silvarrey, L.S.; Phan, A.N. Kinetic study of municipal plastic waste. Int. J. Hydrogen Energy 2016, 41, 16352–16364. [Google Scholar] [CrossRef] [Green Version]
- Cardona, S.C.; Corma, A. Tertiary recycling of polypropylene by catalytic cracking in a semi batch stirred reactor. Appl. Catalysis B 2000, 25, 151–162. [Google Scholar] [CrossRef]
- Arandes, J.; Abajo, I.; Lopez-Valerio, D.; Fernandez, I.; Azkoiti, M.J.; Olazar, M.; Bilbao, J. Transformation of several plastic wastes into fuels by catalytic cracking. Ind. Eng. Chem. Res. 1997, 36, 4523–4529. [Google Scholar] [CrossRef]
- Kaminsky, W.; Schlesselmann, B.; Simon, C.M. Thermal degradation of mixed plastic waste to aromatic and gas. Poly. Degrad. Stab. 1996, 53, 189–197. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Miguel, G.S.; Castro, M.C.; Madrid, S. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Jan, M.R.; Shah, J.; Gulab, H. Catalytic conversion of waste high-density polyethylene into useful hydrocarbons. Fuel 2013, 105, 595–602. [Google Scholar] [CrossRef]
- Lyon, R.E. An integral method of non-isothermal kinetic analysis. Thermochim. Acta 1997, 297, 117–124. [Google Scholar] [CrossRef]
- Saha, B.; Ghoshal, A.K. Model-free kinetics analysis of ZSM-5 catalyzed pyrolysis of waste LDPE. Thermochim. Acta 2007, 453, 120–127. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; Bouadili, A. Pyrolysis of olive residue/low density polyethylene mixture: Part I Thermogravimetric kinetics. J. Fuel Chem. Technol. 2008, 36, 672–678. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Aguado, R.; Olaza, M.; Gaisan, B.; Prieto, R.; Bilbao, J. Kinetic study of polyolefin pyrolysis in a conical spouted bed reactor, Chem. Eng. J. 2003, 92, 91–99. [Google Scholar] [CrossRef]
- Sorum, L.; Gronli, M.; Hustad, J.E. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.Y.; Hor, J.L.; Shih, S.M.; Chen, L.W.; Chang, F.W. On the thermal treatment of plastic mixture of MSW: Pyrolysis kinetics. Waste Manag. 1993, 13, 221–235. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Conesa, J.A.; Caballero, J.A.; Reyes-Labarta, A.J. Artificial neural network for modelling thermal decompositions. J. Anal. Appl. Pyrolysis 2004, 71, 343–352. [Google Scholar] [CrossRef]
- Yıldız, Z.; Uzun, H.; Ceylan, S.; Topcu, Y. Application of artificial neural networks to co-combustion of hazelnut husk–lignite coal blends. Bioresour. Technol. 2016, 200, 42–47. [Google Scholar] [CrossRef]
- Çepelioĝullar, O.; Mutlu, I.; Yaman, S.; Haykiri-Acma, H. A study to predict pyrolytic behaviors of refuse-derived fuel (RDF): Artificial neural network application. J. Anal. Appl. Pyrolysis 2016, 122, 84–94. [Google Scholar] [CrossRef]
- Charde, S.J.; Sonawane, S.S.; Sonawane, S.H.; Shimpi, N.G. Degradation Kinetics of Polycarbonate Composites: Kinetic Parameters and Artificial Neural Network. Chem. Biochem. Eng. Q. 2018, 32, 151–165. [Google Scholar] [CrossRef]
- Chen, J.; Xie, C.; Liu, J.; He, Y.; Xie, W.; Zhang, X.; Chang, K.; Kuo, J.; Sun, J.; Zheng, L.; et al. Co-combustion of sewage sludge and coffee grounds under increased O2/CO2 atmospheres: Thermodynamic characteristics, kinetics and artificial neural network modeling. Bioresour. Technol. 2018, 250, 230–238. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Taqvi, S.A.; Naqvi, M.; Niazi, M.B.K.; Noor, T.; Farooq, W. Pyrolysis of high-ash sewage sludge: Thermo-kinetic study using TGA and artificial neural networks. Fuel 2018, 233, 529–538. [Google Scholar] [CrossRef]
- Chan, J.H.; Balke, S.T. The thermal degradation kinetics of polypropylene: Part III. thermogravimetric analyses. Polym. Degrad. Stabil. 1997, 57, 135–149. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stabil. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tiana, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Gundogar, A.S.; Kok, M.V. Thermal characterization, combustion and kinetics of different crude oils. Fuel 2014, 123, 59–65. [Google Scholar] [CrossRef]
- Baloch, M.K.; Khurran, M.J.Z.; Durrani, G.F. Application of different methods for the Thermogravimetric analysis of polyethylene samples. J. Appl. Polym. Sci. 2011, 120, 3511–3518. [Google Scholar] [CrossRef]
- Quantrille, T.E.; Liu, Y.A. Artificial Intelligence in Chemical Engineering; Academic Press: San Diego, LA, USA, 1991. [Google Scholar]
- Halali, M.A.; Azari, V.; Arabloo, M.; Mohammadi, A.H.; Bahadori, A. Application of a radial basis function neural network to estimate pressure gradient in water–oil pipelines. J. Taiwan Inst. Chem. Eng. 2016, 58, 189–202. [Google Scholar] [CrossRef]
- Govindan, B.; Jakka, S.C.B.; Radhakrishnan, T.K.; Tiwari, A.K.; Sudhakar, T.M.; Shanmugavelu, P.; Kalburgi, A.K.; Sanyal, A.; Sarkar, S. Investigation on kinetic parameters of combustion and oxy-combustion of calcined pet coke employing thermogravimetric analysis coupled to artificial neural network modeling. Energy Fuels 2018, 32, 3995–4007. [Google Scholar] [CrossRef]
- Boostani, M.; Karimi, H.; Azizi, S. Heat transfer to oil-water flow in horizontal and inclined pipes: Experimental investigation and ANN modeling. Int. J. Therm. Sci. 2017, 111, 340–350. [Google Scholar] [CrossRef]
- Beale, M.H.; Hagan, M.T.; Demuth, H.B. Neural Network Toolbox TM User’s Guide; MathWorks: Natick, MA, USA, 2018. [Google Scholar]
- Quan, B.; Kun, C.; Wei, X.; Yuanyuan, L.; Mengjie, C.; Xianghai, K.; Qiulu, C.; Hanping, M. Hydrocarbon rich bio-oil production, thermal behavior analysis and kinetic study of microwave-assisted co-pyrolysis of microwave-torrefied lignin with low density polyethylene. Bioresour. Technol. 2019, 291, 121860. [Google Scholar] [CrossRef]








| Reference | Activation Energy (kJ mol−1) |
|---|---|
| Diaz Silvarrey and Phan [2] | 267.61 ± 3.23 |
| Lyon [8] | 130–200 |
| Saha and Ghoshal [9] | 190 |
| Aboulkas et al. [10] | 215 |
| Aboulkas et al. [11] | 215–221 |
| Aguado et al. [12] | 261 ± 21 |
| Sorum et al. [13] | 340 |
| Wu et al. [14] | 194–206 |
| Manufacturer | Ipoh SY Recycle Plastic, Perak, Malaysia |
|---|---|
| Polymer Type | Recycled LDPE |
| Appearance (at 25 °C) | Solid |
| Physical State | Pellets |
| Colour | Black |
| Density (Kg/m3) | 910–940 |
| Melting Temperature (°C) | 115 ± 10 |
| Method | Equation | Integral (I) or Differential (D) | Plot | |
|---|---|---|---|---|
| Friedman | (6) | D | ||
| Flynn-Wall-Qzawa (FWO) | (7) | I | ||
| Kissinger-Akahira-Sunose (KAS) | (8) | I | ||
| Method | Equation | Plot | |
|---|---|---|---|
| Arrhenius | (9) | ||
| Coats-Redfern | n ≠ 1 | (10) | |
| n = 1 | (11) | ||
| Heating Rate (K/min) | On-Set (K) | End-Set (K) | Peak (K) |
|---|---|---|---|
| 5 | 665 | 750 | 741 |
| 10 | 668 | 755 | 744 |
| 20 | 688 | 782 | 765 |
| 40 | 700 | 794 | 785 |
| Conversion | Friedman | FWO | KAS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E (kJ/mol) | A (min−1) | R2 | E (kJ/mol) | A (min−1) | R2 | E (kJ/mol) | A (min−1) | R2 | |
| 0.1 | 197 | 2.63 × 1013 | 0.9772 | 193 | 8.14 × 1012 | 0.9532 | 191 | 5.51 × 1012 | 0.9474 |
| 0.2 | 185 | 4.85 × 1012 | 0.9265 | 198 | 2.17 × 1013 | 0.9575 | 196 | 1.49 × 1013 | 0.9523 |
| 0.3 | 186 | 6.68 × 1012 | 0.9288 | 198 | 2.46 × 1013 | 0.9629 | 196 | 1.65 × 1013 | 0.9582 |
| 0.4 | 206 | 1.97 × 1014 | 0.9387 | 195 | 1.70 × 1013 | 0.9498 | 193 | 1.09 × 1013 | 0.9435 |
| 0.5 | 198 | 5.20 × 1013 | 0.9793 | 194 | 1.55 × 1013 | 0.9527 | 191 | 9.74 × 1012 | 0.9467 |
| 0.6 | 194 | 3.06 × 1013 | 0.9844 | 194 | 1.97 × 1013 | 0.9567 | 192 | 1.23 × 1013 | 0.9511 |
| 0.7 | 188 | 1.17 × 1013 | 0.9674 | 196 | 3.07 × 1013 | 0.9612 | 194 | 1.94 × 1013 | 0.9562 |
| 0.8 | 196 | 4.31 × 1013 | 0.9345 | 197 | 4.15 × 1013 | 0.9665 | 195 | 2.62 × 1013 | 0.9621 |
| 0.9 | 198 | 4.50 × 1013 | 0.9559 | 192 | 2.16 × 1013 | 0.9720 | 190 | 1.28 × 1013 | 0.9681 |
| Average | 194 | 4.63 × 1013 | 0.9547 | 195 | 2.23 × 1013 | 0.9592 | 193 | 1.43 × 1013 | 0.9540 |
| Heating Rate (K/min) | Arrhenius Method | Coats-Redfern Method | ||||
|---|---|---|---|---|---|---|
| E (kJ/mol) | A (min−1) | R2 | E (kJ/mol) | A (min−1) | R2 | |
| 5 | 207 | 1.42 × 1014 | 0.9673 | 193 | 4.22 × 1010 | 0.9295 |
| 10 | 200 | 2.29 × 1013 | 0.985 | 193 | 6.75 × 1010 | 0.9436 |
| 20 | 213 | 9.13 × 1013 | 0.9724 | 197 | 8.66 × 1010 | 0.9413 |
| 40 | 187 | 1.11 × 1012 | 0.9649 | 201 | 1.61 × 1011 | 0.9459 |
| Average | 202 | 6.43 × 1013 | 0.9724 | 196 | 8.92 × 1010 | 0.9401 |
| Model | Network Topology | 1st Transfer Function | 2nd Transfer Function | R |
|---|---|---|---|---|
| ANN1 | NN-2-10-1 | TANSIG | - | 0.99943 |
| ANN2 | NN-2-15-1 | TANSIG | - | 0.99981 |
| ANN3 | NN-2-5-1 | TANSIG | - | 0.99724 |
| ANN4 | NN-2-10-1 | LOGSIG | - | 0.99865 |
| ANN5 | NN-2-15-1 | LOGSIG | - | 0.98047 |
| ANN6 | NN-2-5-1 | LOGSIG | - | 0.99544 |
| ANN7 | NN-2-15-15-1 | TANSIG | TANSIG | 0.99978 |
| ANN8 | NN-2-15-15-1 | LOGSIG | TANSIG | 0.99961 |
| ANN9 | NN-2-15-15-1 | TANSIG | LOGSIG | 0.99989 |
| ANN10 | NN-2-10-15-1 | TANSIG | LOGSIG | 0.99990 |
| ANN11 | NN-2-10-10-1 | TANSIG | LOGSIG | 0.99993 |
| ANN12 | NN-2-10-10-1 | LOGSIG | LOGSIG | 1.00000 |
| ANN13 | NN-2-15-15-1 | LOGSIG | LOGSIG | 0.99998 |
| ANN14 | NN-2-10-15-1 | LOGSIG | LOGSIG | 0.99997 |
| ANN15 | NN-2-15-10-1 | LOGSIG | LOGSIG | 0.99996 |
| Set | Statistical Parameters | |||
|---|---|---|---|---|
| R | RMSE | MAE | MBE | |
| Training | 0.99999 | 0.09786 | 0.04177 | 0.00583 |
| Validation | 0.99999 | 0.04578 | 0.03291 | −0.01063 |
| Test | 0.99999 | 0.05197 | 0.03713 | 0.002655 |
| All | 0.99999 | 0.08621 | 0.03975 | 0.002897 |
| No. | Input Data | Predicted-Output Data | |
|---|---|---|---|
| Heating Rate (K min−1) | Temperature (K) | Weight Left (%) | |
| 1 | 5 | 528.036 | 99.87579 |
| 2 | 5 | 578.09 | 99.6904 |
| 3 | 5 | 628.072 | 99.328 |
| 4 | 5 | 678.062 | 96.50681 |
| 5 | 5 | 728.025 | 49.30348 |
| 6 | 5 | 778.043 | 0.048376 |
| 7 | 5 | 828.05 | −0.01156 |
| 8 | 10 | 528.014 | 100.0249 |
| 9 | 10 | 578.026 | 99.66833 |
| 10 | 10 | 628.017 | 98.99761 |
| 11 | 10 | 678 | 96.5807 |
| 12 | 10 | 728.002 | 64.78094 |
| 13 | 10 | 778 | 0.450783 |
| 14 | 10 | 828.018 | 0.344112 |
| 15 | 20 | 528.148 | 99.97255 |
| 16 | 20 | 578.205 | 99.85724 |
| 17 | 20 | 628.006 | 99.64173 |
| 18 | 20 | 678.273 | 98.72066 |
| 19 | 20 | 728.203 | 88.68082 |
| 20 | 20 | 778.291 | 0.577601 |
| 21 | 20 | 828.075 | −0.04285 |
| 22 | 40 | 528.194 | 99.98355 |
| 23 | 40 | 578.397 | 99.8972 |
| 24 | 40 | 628.452 | 99.74232 |
| 25 | 40 | 678.12 | 99.26672 |
| 26 | 40 | 728.501 | 94.30099 |
| 27 | 40 | 778.047 | 30.7278 |
| 28 | 40 | 828.38 | 0.264529 |
| Set | Statistical Parameters | |||
|---|---|---|---|---|
| R | RMSE | MAE | MBE | |
| simulated | 0.99998 | 0.17017 | 0.07941 | 0.04903 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubdub, I.; Al-Yaari, M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers 2020, 12, 891. https://doi.org/10.3390/polym12040891
Dubdub I, Al-Yaari M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers. 2020; 12(4):891. https://doi.org/10.3390/polym12040891
Chicago/Turabian StyleDubdub, Ibrahim, and Mohammed Al-Yaari. 2020. "Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction" Polymers 12, no. 4: 891. https://doi.org/10.3390/polym12040891
APA StyleDubdub, I., & Al-Yaari, M. (2020). Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers, 12(4), 891. https://doi.org/10.3390/polym12040891