Poly (vinyl alcohol)/β-Cyclodextrin Composite Fiber with Good Flame Retardant and Super-Smoke Suppression Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
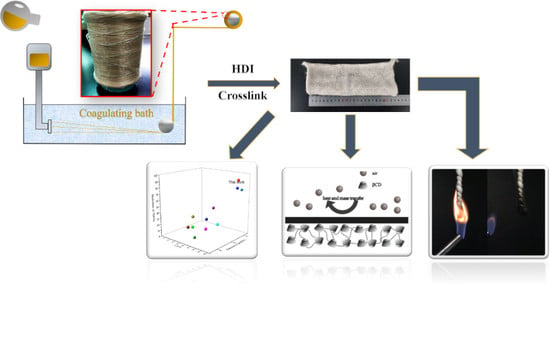
2.2. Preparation of PVA and PVA/CD Fibers
2.3. Preparation of Crosslinked PVA and PVA/CD Fibers
2.4. Characterization
3. Results
3.1. Preparation of PVA/CD/HDI Fibers
3.2. Mechanical Properties of PVA/CD/HDI Fibers
3.3. Flame Retardant Assessment
3.4. Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pethsangave, D.A.; Khose, R.V.; Wadekar, P.H.; Some, S. Deep eutectic solvent functionalized graphene composite as an extremely high potency flame retardant. ACS Appl. Mater. Interfaces 2017, 9, 35319–35324. [Google Scholar] [CrossRef]
- Li, Y.C.; Mannen, S.; Morgan, A.B.; Chang, S.; Yang, Y.H.; Condon, B.; Grunlan, J.C. Intumescent all-polymer multilayer nanocoating capable of extinguishing flame on fabric. Adv. Mater. 2011, 23, 3926–3931. [Google Scholar] [CrossRef]
- Shi, Y.; Long, Z.; Yu, B.; Zhou, K.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Tunable thermal, flame retardant and toxic effluent suppression properties of polystyrene based on alternating graphitic carbon nitride and multi-walled carbon nanotubes. J. Mater. Chem. A 2015, 3, 17064–17073. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Rużycka, M.; Wroczyński, P.; Purser, D.A.; Stec, A. Analysis of fire feaths in Poland and influence of smoke toxicity. Forensic. Sci. Int. 2017, 277, 77–87. [Google Scholar] [CrossRef]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef]
- Ryu, B.Y.; Emrick, T. Thermally induced structural transformation of bisphenol-1, 2, 3-triazole polymers: Smart, self-extinguishing materials. Angew. Chem. Int. Ed. 2010, 49, 9644–9647. [Google Scholar] [CrossRef]
- Van den Eede, N.; Heffernan, A.L.; Aylward, L.L.; Hobson, P.; Neels, H.; Mueller, J.F.; Covaci, A. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 2015, 74, 1–8. [Google Scholar] [CrossRef]
- Reti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay–chitosan nanobrick walls: Completely renewable gas barrier and flame-retardant nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643–1649. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame retardant properties of isosorbide bis-phosphorus esters. Polym. Degrad. Stabil. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Qian, W.; Li, X.Z.; Wu, Z.P.; Liu, Y.X.; Fang, C.C.; Meng, W. Formulation of intumescent flame retardant coatings containing natural-based tea saponin. J. Agric. Food. Chem. 2015, 63, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, M.; Guan, J.P.; Tang, R.C.; Qiao, Y.F. Casein phosphopeptide-metal salts combination: A novel route for imparting the durable flame retardancy to silk. J. Taiwan Inst. Chem. E 2019, 101, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, D.; Alonso, J.P.; Wang, D.Y. Inclusion complex between beta-cyclodextrin and phenylphosphonicdiamide as novel bio-based flame retardant to epoxy: Inclusion behavior, characterization and flammability. Mater. Des. 2017, 114, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, X.Y.; Cheng, X.W.; Tang, R.C. Phytic acid as an eco-friendly flame retardant for silk/wool blend: A comparative study with fluorotitanate and fluorozirconate. J. Clean. Prod. 2018, 198, 1044–1052. [Google Scholar] [CrossRef]
- Kurańska, M.; Cabulis, U.; Auguścik, M.; Prociak, A.; Ryszkowska, J.; Kirpluks, M. Bio-based polyurethane-polyisocyanurate composites with an intumescent flame retardant. Polym. Degrad. Stabil. 2016, 127, 11–19. [Google Scholar] [CrossRef]
- Tissot, I.; Novat, C.; Lefebvre, F.; Bourgeat-Lami, E. Hybrid latex particles coated with silica. Macromolecules 2001, 34, 5737–5739. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Bai, L.; Du, R.; Liu, Y.; Zhang, Y.; Zhao, G. Effect of micro-Al2O3 contents on mechanical property of carbon fiber reinforced epoxy matrix composites. Compos. Part. B-Eng. 2016, 91, 392–398. [Google Scholar] [CrossRef]
- Wang, B.; Qian, X.; Shi, Y.; Yu, B.; Hong, N.; Song, L.; Hu, Y. Cyclodextrin microencapsulated ammonium polyphosphate: Preparation and its performance on the thermal, flame retardancy and mechanical properties of ethylene vinyl acetate copolymer. Compos. Part. B-Eng. 2015, 69, 22–30. [Google Scholar] [CrossRef]
- Han, F.; Liu, Q.; Lai, X.; Li, H.; Zeng, X. Compatibilizing effect of β-cyclodextrin in RDP/phosphorus -containing polyacrylate composite emulsion and its synergism on the flame retardancy of the latex film. Prog. Org. Coat. 2014, 77, 975–980. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, L.; Liu, Y.; Wang, H.A. Facile and novel modification method of β-cyclodextrin and its application in intumescent flame-retarding polypropylene with melamine phosphate and expandable graphite. J. Polym. Res. 2016, 23, 74–91. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, X.; Ma, S.; Xiong, Z.; Zhang, C.; Jiang, Y.; Zhu, J. Syntheses of metallic cyclodextrins and their use as synergists in a poly (vinyl alcohol)/intumescent flame retardant system. Ind. Eng. Chem. Res. 2013, 52, 2784–2792. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, J.; Pasquinelli, M.A.; Hinks, D.; Tonelli, A.E. Formation and characterization of an inclusion complex of triphenyl phosphate and β-cyclodextrin and its use as a flame retardant for polyethylene terephthalate. Polym. Degrad. Stabil. 2015, 120, 244–250. [Google Scholar] [CrossRef]
- Jeddi, M.K.; Laitinen, O.; Liimatainen, H. Magnetic superabsorbents based on nanocellulose aerobeads for selective removal of oils and organic solvents. Mater. Des. 2019, 183, 108115. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Xing, C.Y.; Guo, K.; Zhang, X.Q.; Ding, L.S.; Zhang, S.; Li, B.J. β-Cyclodextrin based air filter for high-efficiency filtration of pollution sources. J. Hazard. Mater. 2019, 373, 197–203. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Forouharshad, M.; Putti, M.; Basso, A.; Prato, M.; Monticelli, O. Biobased system composed of electrospun sc-PLA/POSS/cyclodextrin fibers to remove water pollutants. ACS Sustain. Chem. Eng. 2015, 3, 2917–2924. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; de Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef]
- Alongi, J.; Poskovic, M.; Visakh, P.M.; Frache, A.; Malucelli, G. Cyclodextrin nanosponges as novel green flame retardants for PP, LLDPE and PA6. Carbohyd. Polym. 2012, 88, 1387–1394. [Google Scholar] [CrossRef]
- Nam, J.; Kim, G.; Lee, B.; Hasegawa, R.; Hama, Y. Frost resistance of polyvinyl alcohol fiber and polypropylene fiber reinforced cementitious composites under freeze thaw cycling. Compos. Part. B-Eng. 2016, 90, 241–250. [Google Scholar] [CrossRef]
- Zhang, X.; Min, B.; Kumar, S. Solution spinning and characterization of poly (vinyl alcohol)/soybean protein blend fibers. J. Appl. Polym. Sci. 2003, 90, 716–721. [Google Scholar] [CrossRef]
- Xu, R.; Wang, C.; Wu, S.; Chen, K. Effects of the polymeric additives on the stickies formation in recycled fibers based papermaking process. Nord. Pulp. Pap. Res. J. 2017, 32, 647–655. [Google Scholar] [CrossRef]
- Wang, Y.; Long, J.; Hu, J.; Sun, Z.; Meng, L. Polyvinyl alcohol/Lyocell dual-layer paper-based separator for primary zinc-air batteries. J. Power Sources 2020, 453, 227853. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, D.; Hu, X.; Ren, N.; Gao, W.; Chen, S.; Chen, H.; Lu, Y.; Bai, Y. Robust and antibacterial polymer/mechanically exfoliated graphene nanocomposite fibers for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, C.; Zhao, X.; Xu, J.; Jiang, M. Melamine formaldehyde/polyvinyl alcohol composite fiber: Structures and properties controlled by reaction-induced phase separation. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, W.; Jiang, M.; Liu, P.; Xu, J. Flame retardant study of formalized polyvinyl alcohol fiber coated with melamine formaldehyde resins and the synergistic effect of copper ions. Polym. Degrad. Stabil. 2017, 144, 331–343. [Google Scholar] [CrossRef]
- Zha, F.; Li, S.; Chang, Y. Preparation and adsorption property of chitosan beads bearing β-cyclodextrin cross-Linked by 1,6-hexamethylene diisocyanate. Carbohyd. Polym. 2008, 72, 456–461. [Google Scholar] [CrossRef]
- Shojaie-Bahaabad, M.; Taheri-Nassaj, E.; Naghizadeh, R. An alumina–YAG nanostructured fiber prepared from an aqueous sol–gel precursor: Preparation, rheological behavior and spinnability. Ceram. Int. 2008, 34, 1893–1902. [Google Scholar] [CrossRef]
- Radishevskii, M.; Serkov, A. Coagulation mechanism in wet spinning of fibers. Fibre. Chem. 2005, 37, 266–271. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Tao, G.; Wang, L.; Zhou, S. Thermo- and water-induced shape memory poly(vinyl alcohol) supramolecular networks crosslinked by self-complementary quadruple hydrogen bonding. Polym. Chem. 2016, 7, 6637–6644. [Google Scholar] [CrossRef]
- Peng, K.; Chen, C.; Pan, W.; Liu, W.; Wang, Z.; Zhu, L. Preparation and properties of β-cyclodextrin/4, 4′-diphenylmethane diisocyanate/polyethylene glycol (β-CD/MDI/PEG) crosslinking copolymers as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 2016, 145, 238–247. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P.; Lopez-Cuesta, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R-Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Z.; Cai, Z.; Yu, L.; Lv, Y. Voriconazole composited polyvinyl alcohol/hydroxypropyl- β-cyclodextrin nanofibers for ophthalmic delivery. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Zanetti, M.; Camino, G. Thermal degradation of cyclodextrins. Polym. Degrad. Stabil. 2000, 69, 373–379. [Google Scholar] [CrossRef]
- Xu, Q.; Jin, C.; Jiang, Y. Compare the flammability of two extruded polystyrene foams with micro-scale combustion calorimeter and cone calorimeter tests. J. Therm. Anal. Calorim. 2017, 127, 2359–2366. [Google Scholar] [CrossRef]
- Tai, Q.; Yuen, R.K.K.; Yang, W.; Qiao, Z.; Song, L.; Hu, Y. Iron-montmorillonite and zinc borate as synergistic agents in flame-retardant glass fiber reinforced polyamide 6 composites in combination with melamine polyphosphate. Compos. Part. A-Appl. Sci. Manuf. 2012, 43, 415–422. [Google Scholar] [CrossRef]
- Feng, J.X.; Su, S.P.; Zhu, J. An intumescent flame retardant system using β-cyclodextrin as a carbon source in polylactic acid (PLA). Polym. Adv. Technol. 2011, 22, 1115–1122. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.W.; Vasagar, V.; Ha, H.S.; Nazarenko, C.; Ellison, J. Polydopamine-graphene oxide flame retardant nanocoatings applied via an aqueous liquid crystalline scaffold. Adv. Funct. Mater. 2018, 28, 1803172. [Google Scholar] [CrossRef]










| Sample | Linear Density/dTex | Breaking Strength/cN/dTex | Elongation at Break/% | Young’s Modulus/cN/dTex |
|---|---|---|---|---|
| PVA | 4.40 | 3.29 ± 0.23 | 30.45 ± 4.37 | 80.68 ± 8.50 |
| PVA/50CD | 6.70 | 2.12 ± 0.13 | 34.15 ± 5.14 | 59.12 ± 6.61 |
| PVA/75CD | 6.90 | 2.01 ± 0.35 | 36.70 ± 6.70 | 55.01 ± 6.34 |
| PVA/100CD | 7.20 | 1.89 ± 0.14 | 38.76 ± 5.19 | 47.80 ± 6.54 |
| PVA/HDI | 5.40 | 2.66 ± 0.26 | 47.74 ± 5.10 | 65.77 ± 10.37 |
| PVA/50CD/HDI | 7.50 | 1.93 ± 0.22 | 56.22 ± 7.26 | 45.51 ± 9.29 |
| PVA/75CD/HDI | 7.80 | 1.60 ± 0.10 | 58.36 ± 7.71 | 39.66 ± 4.40 |
| PVA/100CD/HDI | 8.10 | 1.45 ± 0.18 | 60.49 ± 10.03 | 37.31 ± 5.19 |
| Sample | LOI/% | UL94 | T1 a/s | T2 b/s | Dripping | Ignite Cotton |
|---|---|---|---|---|---|---|
| PVA | 18.8 ± 0.2 | Failed | - | - | No | No |
| PVA/75CD | 21.9 ± 0.3 | V1 | 10.6 ± 3.4 | 45.8 ± 15.9 | No | No |
| PVA/HDI | 27.9 ± 0.3 | V0 | 0.4 ± 0.9 | 2.8± 1.9 | No | No |
| PVA/75CD/HDI | 41.7 ± 0.4 | V0 | 0 ± 0 | 0 ± 0 | No | No |
| Sample | PVA | PVA/75CD | PVA/HDI | PVA/75CD/HDI |
|---|---|---|---|---|
| TTI/s | 35 | 19 | 17 | 66 |
| PHRR/kW/m2 | 355 | 257 | 187 | 79 |
| THR/MJ/kg | 33 | 38 | 38 | 16 |
| avSEA/m2/kg | 704 | 373 | 676 | 188 |
| avMLR/mg/s | 65 | 62 | 53 | 35 |
| TSR/m2/m2 | 1550 | 898 | 1795 | 399 |
| TSP/m2 | 14 | 8 | 16 | 4 |
| Char residue/% | 1.78 | 3.85 | 2.38 | 13.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, C.-Y.; Zeng, S.-L.; Qi, S.-K.; Jiang, M.-J.; Xu, L.; Chen, L.; Zhang, S.; Li, B.-J. Poly (vinyl alcohol)/β-Cyclodextrin Composite Fiber with Good Flame Retardant and Super-Smoke Suppression Properties. Polymers 2020, 12, 1078. https://doi.org/10.3390/polym12051078
Xing C-Y, Zeng S-L, Qi S-K, Jiang M-J, Xu L, Chen L, Zhang S, Li B-J. Poly (vinyl alcohol)/β-Cyclodextrin Composite Fiber with Good Flame Retardant and Super-Smoke Suppression Properties. Polymers. 2020; 12(5):1078. https://doi.org/10.3390/polym12051078
Chicago/Turabian StyleXing, Cheng-Yuan, Shi-Lin Zeng, Shi-Kai Qi, Meng-Jin Jiang, Long Xu, Li Chen, Sheng Zhang, and Bang-Jing Li. 2020. "Poly (vinyl alcohol)/β-Cyclodextrin Composite Fiber with Good Flame Retardant and Super-Smoke Suppression Properties" Polymers 12, no. 5: 1078. https://doi.org/10.3390/polym12051078
APA StyleXing, C. -Y., Zeng, S. -L., Qi, S. -K., Jiang, M. -J., Xu, L., Chen, L., Zhang, S., & Li, B. -J. (2020). Poly (vinyl alcohol)/β-Cyclodextrin Composite Fiber with Good Flame Retardant and Super-Smoke Suppression Properties. Polymers, 12(5), 1078. https://doi.org/10.3390/polym12051078