Graphene Oxide Composite for Selective Recognition, Capturing, Photothermal Killing of Bacteria over Mammalian Cells
Abstract
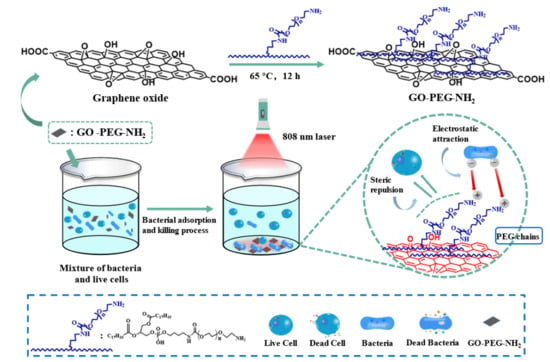
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Synthesis of GO-PEG-NH2
2.3. Preparation of Bacterial Solutions
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. ζ Potential Measurements
2.7. Antibacterial Experiments
2.8. Scanning Electron Microscopy (SEM) Characterization
2.9. E. coli was Induced by IPTG to Express Recombinant Green Fluorescent Protein
2.10. Confocal Laser Scanning Microscopy (CLSM) Characterization
3. Results and Discussion
3.1. Preparation and Mechanism of GO-PEG-NH2
3.2. Characterization of Structure and Photothermal Properties
3.3. Selective Adsorption of GO-PEG-NH2
3.4. Antibacterial Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallagher, L.A.; Shendure, J.; Manoil, C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. MBio 2011, 2, e00315-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sortino, S. Photoactivated nanomaterials for biomedical release applications. J. Mater. Chem. 2012, 22, 301–318. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Koo, H.; Lee, D.E.; Choi, K.; Kwon, I.C.; Kim, K. Multifunctional Chitosan Nanoparticles for Tumor Imaging and Therapy. Adv. Polym. Sci. 2011, 243, 139–161. [Google Scholar]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2014, 8, 340–354. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mao, H.; Wan, Z.; Zhu, A.; Guo, M.; Li, Y.; Li, X.; Wan, J.; Yang, X.; Shuai, X.; et al. Micelles assembled with carbocyanine dyes for theranostic near-infrared fluorescent cancer imaging and photothermal therapy. Biomaterials 2013, 34, 9124–9133. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Kim, H.; Chung, K.; Lee, S.; Kim, D.H.; Lee, H. Near-infrared light-responsive nanomaterials for cancer theranostics. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2016, 8, 23–45. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, H.; Bai, H.; Zhang, P.; Lv, F.; Liu, L.; Dai, Z.; Bao, J.; Wang, S. Electrochemiluminescence for Electric-Driven Antibacterial Therapeutics. J. Am. Chem. Soc. 2018, 140, 2284–2291. [Google Scholar] [CrossRef]
- Bai, H.; Yuan, H.; Nie, C.; Wang, B.; Lv, F.; Liu, L.; Wang, S. A Supramolecular Antibiotic Switch for Antibacterial Regulation. Angew. Chem. Int. Ed. Engl. 2015, 54, 13208–13213. [Google Scholar] [CrossRef]
- Xing, C.; Xu, Q.; Tang, H.; Liu, L.; Wang, S. Conjugated Polymer/Porphyrin Complexes for Efficient Energy Transfer and Improving Light-Activated Antibacterial Activity. J. Am. Chem. Soc. 2009, 131, 13117–13124. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Ma, Y.H.; Gao, G.; Chen, X.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xing, Y.; Wang, R.; Yu, F.; Yu, F. Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer. ACS Appl. Bio Mater. 2019, 3, 86–106. [Google Scholar] [CrossRef]
- Peng, R.; Luo, Y.; Cui, Q.; Wang, J.; Li, L. Near-Infrared Conjugated Oligomer for Effective Killing of Bacterial through Combination of Photodynamic and Photothermal Treatment. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Q.; Liu, L.; Lv, F.; Li, S.; Yang, G.; Wang, S. Multifunctional cationic poly(p-phenylene vinylene) polyelectrolytes for selective recognition, imaging, and killing of bacteria over mammalian cells. Adv. Mater. 2011, 23, 4805–4810. [Google Scholar] [CrossRef]
- Gao, M.; Hu, Q.; Feng, G.; Tomczak, N.; Liu, R.; Xing, B.; Tang, B.Z.; Liu, B. A multifunctional probe with aggregation-induced emission characteristics for selective fluorescence imaging and photodynamic killing of bacteria over mammalian cells. Adv. Healthc Mater. 2015, 4, 659–663. [Google Scholar] [CrossRef]
- Chen, Q.; Wen, J.; Li, H.; Xu, Y.; Liu, F.; Sun, S. Recent advances in different modal imaging-guided photothermal therapy. Biomaterials 2016, 106, 144–166. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, P.; Wang, Y.; Bai, H.; Wang, S.; Xu, J.F.; Zhang, X. Supramolecular Radical Anions Triggered by Bacteria In Situ for Selective Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 16239–16242. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ji, X.; Xu, X.; Islam, M.A.; Li, Z.; Chen, S.; Saw, P.E.; Zhang, H.; Bharwani, Z.; Guo, Z.; et al. Antimonene Quantum Dots: Synthesis and Application as Near-Infrared Photothermal Agents for Effective Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 11896–11900. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martinez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.K.; Yu, X.F.; Wang, J.H.; Li, Z.B.; Li, P.H.; Wang, H.; Song, L.; Chu, P.K.; Li, C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 2016, 78, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Liu, H.; Wang, Y.; Liu, L.; Lv, F.; Li, Y.; Wang, S. Graphdiyne Materials as Nanotransducer for in Vivo Photoacoustic Imaging and Photothermal Therapy of Tumor. Chem. Mater. 2017, 29, 6087–6094. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Zhang, J.; Ma, R.; Gao, J.; Liu, Y.; Zhang, C.; Zhang, Z. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials 2014, 35, 5847–5861. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H.; et al. PEGylated WS(2) nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Deb, A.; Vimala, R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Li, D.; Gao, D.; Qi, J.; Chai, R.; Zhan, Y.; Xing, C. Conjugated Polymer/Graphene Oxide Complexes for Photothermal Activation of DNA Unzipping and Binding to Protein. ACS Appl. Bio Mater. 2018, 1, 146–152. [Google Scholar] [CrossRef]
- Wei, W.; Qu, X. Extraordinary physical properties of functionalized graphene. Small 2012, 8, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lv, W.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. A graphene-based smart catalytic system with superior catalytic performances and temperature responsive catalytic behaviors. Nanoscale 2013, 5, 6275–6279. [Google Scholar] [CrossRef] [Green Version]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; Garcia, H. Active sites on graphene-based materials as metal-free catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef] [Green Version]
- Gerber, I.C.; Serp, P. A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, B.; Wu, K.-H.; Su, D.; Qi, W. Surface chemistry of nanocarbon: Characterization strategies from the viewpoint of catalysis and energy conversion. Carbon 2019, 143, 915–936. [Google Scholar] [CrossRef]
- Martí, M.; Frígols, B.; Salesa, B.; Serrano-Aroca, Á. Calcium alginate/graphene oxide films: Reinforced composites able to prevent Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections with no cytotoxicity for human keratinocyte HaCaT cells. Eur. Polym. J. 2019, 110, 14–21. [Google Scholar] [CrossRef]
- Salesa, B.; Marti, M.; Frigols, B.; Serrano-Aroca, A. Carbon Nanofibers in Pure Form and in Calcium Alginate Composites Films: New Cost-Effective Antibacterial Biomaterials against the Life-Threatening Multidrug-Resistant Staphylococcus epidermidis. Polymers (Basel) 2019, 11, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Tan, S.; Wu, X.; Xing, Y.; Lilak, S.; Wu, M.; Zhao, J.X. Enhanced synergetic antibacterial activity by a reduce graphene oxide/Ag nanocomposite through the photothermal effect. Colloids Surf. B Biointerfaces 2020, 185, 110616. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Auletta, J.T.; Huang, Z.; Chen, X.; Xia, F.; Yang, S.; Liu, H.; Yang, L. Surface Disinfection Enabled by a Layer-by-Layer Thin Film of Polyelectrolyte-Stabilized Reduced Graphene Oxide upon Solar Near-Infrared Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 10511–10517. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, L.; Wang, G.; Yang, K.; Chen, M.; Tian, R.; Ma, Q.; Zhu, L. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 2016, 79, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Su, Y.L.; Hu, S.H.; Chen, S.Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, D.; Song, S.; Wang, X.; Zhang, H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 2013, 34, 7715–7724. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Horváth, L.; Magrez, A.; Burghard, M.; Kern, K.; Forró, L.; Schwaller, B. Evaluation of the toxicity of graphene derivatives on cells of the lung luminal surface. Carbon 2013, 64, 45–60. [Google Scholar] [CrossRef] [Green Version]
- Zhen, X.; Xie, C.; Jiang, Y.; Ai, X.; Xing, B.; Pu, K. Semiconducting Photothermal Nanoagonist for Remote-Controlled Specific Cancer Therapy. Nano Lett. 2018, 18, 1498–1505. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, Y.; Shi, S.; Hao, S.; Wei, J.; Chen, X. Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy. Chem. Asian J. 2015, 10, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, H.; Pu, K. Nanotransducers for Near-Infrared Photoregulation in Biomedicine. Adv. Mater. 2019, 31, e1901607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Q.; Liu, L.; Wang, S. A potent fluorescent probe for the detection of cell apoptosis. Chem Commun (Camb) 2011, 47, 5524–5526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Fu, Y.-Y.; Wu, L.-J.; Li, J.; Yang, H.-H.; Chen, G.-N. Targeted photothermal ablation of pathogenic bacterium, Staphylococcus aureus, with nanoscale reduced graphene oxide. J. Mater. Chem. B 2013, 1, 2496. [Google Scholar] [CrossRef]
- Jia, X.; Ahmad, I.; Yang, R.; Wang, C. Versatile graphene-based photothermal nanocomposites for effectively capturing and killing bacteria, and for destroying bacterial biofilms. J. Mater. Chem. B 2017, 5, 2459–2467. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Kempaiah, R.; Huang, P.J.; Maheshwari, V.; Liu, J. Adsorption and desorption of DNA on graphene oxide studied by fluorescently labeled oligonucleotides. Langmuir 2011, 27, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. Acs Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. Acs Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]





| Species | ζ Potential/mV | ζ Potential/mV |
|---|---|---|
| GO-PEG-NH2 (−) | GO-PEG-NH2 (+) | |
| GO | −41.30 ± 0.36 | - |
| GO-PEG-NH2 | −4.27 ± 0.52 | - |
| E. coli | −51.83 ± 0.61 | −14.60 ± 0.79 |
| S. aureus | −34.63 ± 0.12 | −25.70 ± 0.91 |
| CCRF-CEM | −11.70 ± 0.16 | −10.80 ± 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Qi, J.; Cui, Q.; Bao, X.; Gao, D.; Xing, C. Graphene Oxide Composite for Selective Recognition, Capturing, Photothermal Killing of Bacteria over Mammalian Cells. Polymers 2020, 12, 1116. https://doi.org/10.3390/polym12051116
Ma G, Qi J, Cui Q, Bao X, Gao D, Xing C. Graphene Oxide Composite for Selective Recognition, Capturing, Photothermal Killing of Bacteria over Mammalian Cells. Polymers. 2020; 12(5):1116. https://doi.org/10.3390/polym12051116
Chicago/Turabian StyleMa, Gang, Junjie Qi, Qifan Cui, Xueying Bao, Dong Gao, and Chengfen Xing. 2020. "Graphene Oxide Composite for Selective Recognition, Capturing, Photothermal Killing of Bacteria over Mammalian Cells" Polymers 12, no. 5: 1116. https://doi.org/10.3390/polym12051116
APA StyleMa, G., Qi, J., Cui, Q., Bao, X., Gao, D., & Xing, C. (2020). Graphene Oxide Composite for Selective Recognition, Capturing, Photothermal Killing of Bacteria over Mammalian Cells. Polymers, 12(5), 1116. https://doi.org/10.3390/polym12051116