Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
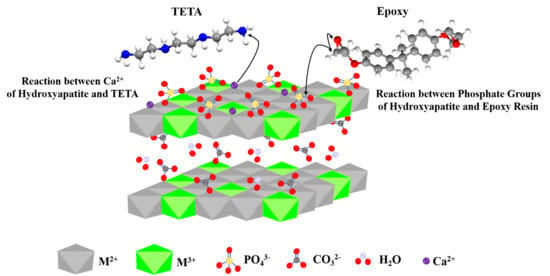
2.2. Synthesis of HA-Functionalized LDHs
2.2.1. Synthesis of Mg-Al-CO3-HA LDH
2.2.2. Synthesis of Zn-Al-CO3-HA LDH
2.3. Preparation of Epoxy/LDH Nanocomposites
2.4. Characterization Methods
3. Results and Discussion
3.1. Characterization of LDHs
3.2. Cure Analysis
3.2.1. Qualitative Description by the Cure Index
3.2.2. Qualitative Cure Assessment by Isoconversional Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Isoconversional Kinetic Methods
Appendix A.1. Friedman Model

Appendix A.2. KAS Method

Appendix A.3. FWO Model

Appendix B. Selection of the Cure Reaction Model
Appendix B.1. Friedman Model

Appendix B.2. Málek Method
| Designation | Heating Rate (°C∙min−1) | αp∞ | αm | αp |
|---|---|---|---|---|
| EP | 2 | 0.4934 | 0.1991 | 0.5188 |
| 5 | 0.4892 | 0.1820 | 0.5435 | |
| 7 | 0.4808 | 0.1860 | 0.5294 | |
| 10 | 0.5606 | 0.2150 | 0.5317 | |
| EP/Zn-Al-CO3-HA | 2 | 0.5098 | 0.0898 | 0.5279 |
| 5 | 0.5601 | 0.0755 | 0.5320 | |
| 7 | 0.4990 | 0.0626 | 0.5229 | |
| 10 | 0.5327 | 0.0408 | 0.5300 | |
| EP/Mg-Al-CO3-HA | 2 | 0.4946 | 0.1161 | 0.5181 |
| 5 | 0.5497 | 0.1032 | 0.5261 | |
| 7 | 0.6362 | 0.0974 | 0.5346 | |
| 10 | 0.5909 | 0.0914 | 0.5337 |

Appendix C. Determination of the Degree of Reaction


References
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z.; et al. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coat. 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Saeb, M.R.; Najafi, F.; Bakhshandeh, E.; Khonakdar, H.A.; Mostafaiyan, M.; Simon, F.; Scheffler, C.; Mäder, E. Highly curable epoxy/MWCNTs nanocomposites: An effective approach to functionalization of carbon nanotubes. Chem. Eng. J. 2015, 259, 117–125. [Google Scholar] [CrossRef]
- Hu, Q.; Memon, H.; Qiu, Y.; Wei, Y. The Failure mechanism of composite stiffener components reinforced with 3D woven fabrics. Materials 2019, 12, 2221. [Google Scholar] [CrossRef] [Green Version]
- Weil, E.D.; Levchik, S. A review of current flame retardant systems for epoxy resins. J. Fire Sci. 2004, 22, 25–40. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Bushy-surface hybrid nanoparticles for developing epoxy superadhesives. Appl. Surf. Sci. 2019, 479, 1148–1160. [Google Scholar] [CrossRef]
- Mohan, P. A critical review: The modification, properties, and applications of epoxy resins. Polym. Plast Tech. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Bukhtiyarova, M. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay. Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Cao, Z.; Li, B.; Sun, L.; Li, L.; Xu, Z.P.; Gu, Z. 2D layered double hydroxide nanoparticles: Recent progress toward preclinical/clinical nanomedicine. Small Methods 2020, 4, 1900343. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)–Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Becker, C.M.; Gabbardo, A.D.; Wypych, F.; Amico, S.C. Mechanical and flame-retardant properties of epoxy/Mg–Al LDH composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Harun, W.; Asri, R.; Alias, J.; Zulkifli, F.; Kadirgama, K.; Ghani, S.; Shariffuddin, J. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Ferraz, M.; Monteiro, F.; Manuel, C. Hydroxyapatite nanoparticles: A review of preparation methodologies. J. Appl. Biomater. Biom. 2004, 2, 74–80. [Google Scholar]
- Zhao, J.-L.; Fu, T.; Han, Y.; Xu, K.-W. Reinforcing hydroxyapatite/thermosetting epoxy composite with 3-D carbon fiber fabric through RTM processing. Mater. Lett. 2004, 58, 163–168. [Google Scholar] [CrossRef]
- Roese, P.B.; Amico, S.C.; Kindlein Júnior, W. Thermal and microestructural characterization of epoxy-infiltrated hydroxyapatite composite. Mater. Res. 2009, 12, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Jouyandeh, M.; Rahmati, N.; Movahedifar, E.; Hadavand, B.S.; Karami, Z.; Ghaffari, M.; Taheri, P.; Bakhshandeh, E.; Vahabi, H.; Ganjali, M.R.; et al. Properties of nano-Fe3O4 incorporated epoxy coatings from Cure Index perspective. Prog. Org. Coat. 2019, 133, 220–228. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, L.; O’Hare, D.; Wang, Q. Thermal properties and flame-retardant characteristics of layered double hydroxide polymer nanocomposites. In Layered Double Hydroxide Polymer Nanocomposites; Elsevier: Cambridge, UK, 2020; pp. 311–345. [Google Scholar]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrion, X.; Laheurte, P.; Vahabi, H.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Jazani, O.M.; Formela, K.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. Curing epoxy resin with anhydride in the presence of halloysite nanotubes: The contradictory effects of filler concentration. Prog. Org. Coat. 2019, 126, 129–135. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Puglia, D.; Saeb, M.R. Epoxy/layered double hydroxide (LDH) nanocomposites: Synthesis, characterization, and Excellent cure feature of nitrate anion intercalated Zn-Al LDH. Prog. Org. Coat. 2019, 136, 105218. [Google Scholar] [CrossRef]
- Karami, Z.; Aghazadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Vahabi, H.; Ganjali, M.R.; Torre, L.; Puglia, D.; Saeb, M.R. Epoxy/Zn-Al-CO3 LDH nanocomposites: Curability assessment. Prog. Org. Coat. 2020, 138, 105355. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ghiyasi, S.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; et al. Exploring curing potential of epoxy nanocomposites containing nitrate anion intercalated Mg–Al–LDH with Cure Index. Prog. Org. Coat. 2020, 139, 105255. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Puglia, D.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Meng, X. Preparation of a Mg/Al/Fe layered supramolecular compound and application for removal of Cr (VI) from laboratory wastewater. RSC Adv. 2017, 7, 34984–34993. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Cui, L.; Ai, S.; Fan, H.; Zhu, L. Electrochemical determination of bisphenol A at Mg–Al–CO3 layered double hydroxide modified glassy carbon electrode. Electrochim. Acta 2010, 55, 603–610. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Development of Mg-Zn-Al-CO3 ternary LDH and its curability in epoxy/amine system. Prog. Org. Coat. 2019, 136, 105264. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah. Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Chen, Q.; Li, H.; Wang, P.; Islam, S.M.; Gu, Q.; Yang, X.; Kanatzidis, M.G. Highly selective and efficient heavy metal capture with polysulfide intercalated layered double hydroxides. J. Mater. Chem. A 2014, 2, 10280–10289. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitão, A.A. Comparative Structural, thermodynamic and electronic analyses of Zn–Al–An− hydrotalcite-like compounds (An− = Cl−, F−, Br−, OH−, CO32− or NO3−): An ab initio study. Appl. Clay. Sci. 2012, 56, 16–22. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Pan, G.; Lundehøj, L.; Nielsen, U.G.; Shi, Y.; Hansen, H.C.B. Phosphate capture by ultrathin MgAl layered double hydroxide nanoparticles. Appl. Clay. Sci. 2019, 177, 82–90. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Thermogravimetric analysis of selected layered double hydroxides. J. Therm. Anal. Calorim. 2013, 112, 649–657. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Dubois, P. Curing epoxy with polyethylene glycol (PEG) surface-functionalized NixFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105250. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Hamad, S.M.; Karimzadeh, I.; Aghazadeh, M.; Karami, Z.; Akbari, V.; Shammiry, F.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; et al. Unconditionally blue: Curing epoxy with polyethylene glycol (PEG) surface-functionalized ZnxFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105285. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Cure Index for labeling curing potential of epoxy/LDH nanocomposites: A case study on nitrate anion intercalated Ni-Al-LDH. Prog. Org. Coat. 2019, 136, 105228. [Google Scholar] [CrossRef]
- Saeb, M.R.; Nonahal, M.; Rastin, H.; Shabanian, M.; Ghaffari, M.; Bahlakeh, G.; Ghiyasi, S.; Khonakdar, H.A.; Goodarzi, V.; Vijayan, P.P.; et al. Calorimetric analysis and molecular dynamics simulation of cure kinetics of epoxy/chitosan-modified Fe3O4 nanocomposites. Prog. Org. Coat. 2017, 112, 176–186. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing kinetics and thermal stability of epoxy composites containing newly obtained nano-scale aluminum hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [Green Version]
- Jouyandeh, M.; Karami, Z.; Hamad, S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Kim, S.-J.; Zarrintaj, P.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/ZnxFe3−xO4 nanocomposites. Prog. Org. Coat. 2019, 136, 105290. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Karimzadeh, I.; Formela, K.; Colom, X.; Cañavate, J.; Saeb, M.R. Curing epoxy with ethylenediaminetetraacetic acid (EDTA) surface-functionalized CoxFe3−xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105248. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional method to explore the mechanism and kinetics of multi-step epoxy cures. Macromol. Rapid Commun. 1999, 20, 387–389. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Miura, K. A new and simple method to estimate f (E) and k0 (E) in the distributed activation energy model from three sets of experimental data. Energy Fuels 1995, 9, 302–307. [Google Scholar] [CrossRef]
- Mashouf Roudsari, G.; Mohanty, A.K.; Misra, M. Study of the curing kinetics of epoxy resins with biobased hardener and epoxidized soybean oil. ACS Sustain. Chem. Eng. 2014, 2, 2111–2116. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Formela, K.; Puglia, D.; Saeb, M.R. Curing behavior of epoxy/Fe3O4 nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coat. 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation. Appl. Surf. Sci. 2018, 447, 152–164. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Khadem, S.S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/MnxFe3−xO4 nanocomposites. Prog. Org. Coat. 2020, 140, 105505. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Zhang, J.; Cao, L.; Dong, H.; Zhang, C.; Xu, X.; Zhu, M.; Li, J. Optimizing curing process of graphene oxide/waterborne epoxy blends by curing kinetics simulation considering the coupling of heat conduction and curing reaction. Thermochim. Acta 2019, 672, 60–69. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Res. Report Chiba Inst. Technol. Sci. Technol. 1971, 16, 22. [Google Scholar]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Vyazovkin, S. Model-free kinetics. J. Therm. Anal. Calorim. 2006, 83, 45–51. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Is the Friedman method applicable to transformations with temperature dependent reaction heat? Macromol. Chem. Phys. 2007, 208, 1592–1597. [Google Scholar] [CrossRef]
- Montserrat, S.; Málek, J. A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 1993, 228, 47–60. [Google Scholar] [CrossRef]
- Tripathi, G.; Srivastava, D. Cure kinetics of ternary blends of epoxy resins studied by nonisothermal DSC data. J. Appl. Polym. Sci. 2009, 112, 3119–3126. [Google Scholar] [CrossRef]
- Roşu, D.; Caşcaval, C.; Mustatǎ, F.; Ciobanu, C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim. Acta 2002, 383, 119–127. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Study of curing kinetics of anhydride cured petroleum-based (DGEBA) epoxy resin and renewable resource based epoxidized soybean oil (ESO) systems catalyzed by 2-methylimidazole. Thermochim. Acta 2017, 654, 112–120. [Google Scholar] [CrossRef]
- Luo, X.; Yu, X.; Ma, Y.; Naito, K.; Zhang, Q. Preparation and cure kinetics of epoxy with nanodiamond modified with liquid crystalline epoxy. Thermochim. Acta 2018, 663, 1–8. [Google Scholar] [CrossRef]
- Zhou, T.; Gu, M.; Jin, Y.; Wang, J. Studying on the curing kinetics of a DGEBA/EMI-2, 4/nano-sized carborundum system with two curing kinetic methods. Polymer 2005, 46, 6174–6181. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Z.; Zou, H.; Liang, M. Curing characteristics of an epoxy resin in the presence of functional graphite oxide with amine-rich surface. Thermochim. Acta 2015, 614, 76–84. [Google Scholar] [CrossRef]









| Sample | Nanoparticle | Content (wt %) |
|---|---|---|
| Ep | - | 0.0 |
| Ep/Zn-Al-CO3-HA | Zn-Al-CO3-HA LDH | 0.1 |
| Ep/Mg-Al-CO3-HA | Mg-Al-CO3-HA LDH | 0.1 |
| Sample | 2θ of Main Peaks | Basal Spacing (C/3) (Å) | Intermetallic Distance (2a) (Å) | ||
|---|---|---|---|---|---|
| (003) | (006) | (110) | |||
| Zn-Al-CO3-HA | 11.17 | 22.12 | 60.28 | 7.91 | 3.06 |
| Mg-Al-CO3-HA | 11.31 | 22.38 | 60.28 | 7.81 | 3.08 |
| Designation | β (°C/min) | Tonset (°C) | Tendset (°C) | Tp (°C) | ΔT (°C) | ΔH∞ (J/g) | ΔT* | ΔH* | CI |
|---|---|---|---|---|---|---|---|---|---|
| EP [21] | 2 | 27.47 | 130.94 | 74.30 | 103.47 | 353.95 | n.a. | n.a. | n.a. |
| 5 | 30.67 | 157.98 | 88.17 | 127.31 | 375.90 | n.a. | n.a. | n.a. | |
| 7 | 33.27 | 172.55 | 95.77 | 139.28 | 345.48 | n.a. | n.a. | n.a. | |
| 10 | 32.22 | 183.55 | 104.41 | 151.32 | 387.63 | n.a. | n.a. | n.a. | |
| EP/Zn-Al-CO3-HA | 2 | 27.86 | 127.39 | 75.28 | 99.53 | 303.06 | 0.96 | 0.85 | 0.82 |
| 5 | 28.72 | 150.21 | 89.14 | 121.49 | 322.93 | 0.95 | 0.86 | 0.82 | |
| 7 | 29.65 | 168.11 | 95.04 | 138.46 | 333.74 | 0.99 | 0.96 | 0.95 | |
| 10 | 33.93 | 172.55 | 101.41 | 138.62 | 381.53 | 0.92 | 0.98 | 0.98 | |
| EP/Mg-Al-CO3-HA | 2 | 30.29 | 126.06 | 74.25 | 95.77 | 292.63 | 0.92 | 0.83 | 0.76 |
| 5 | 32.72 | 150.61 | 89.62 | 117.89 | 361.07 | 0.93 | 0.96 | 0.89 | |
| 7 | 35.45 | 149.76 | 95.00 | 114.31 | 309.27 | 0.82 | 0.89 | 0.73 | |
| 10 | 35.94 | 168.25 | 102.18 | 132.31 | 341.76 | 0.87 | 0.88 | 0.76 |
| Designation | Heating Rate (°C∙min−1) | Eα (kJ/mol) | ln(A) (1/s) | Mean (1/s) | m | Mean | n | Mean |
|---|---|---|---|---|---|---|---|---|
| Friedman method | ||||||||
| EP | 2 | 49.38 | 15.44 | 15.52 | 0.44 | 0.44 | 1.40 | 1.42 |
| 5 | 15.64 | 0.44 | 1.41 | |||||
| 7 | 15.48 | 0.39 | 1.42 | |||||
| 10 | 15.55 | 0.49 | 1.46 | |||||
| EP/Zn-Al-CO3-HA | 2 | 55.41 | 17.21 | 17.23 | 0.23 | 0.21 | 1.35 | 1.38 |
| 5 | 17.22 | 0.19 | 1.34 | |||||
| 7 | 17.23 | 0.21 | 1.42 | |||||
| 10 | 17.26 | 0.21 | 1.42 | |||||
| EP/Mg-Al-CO3-HA | 2 | 55.61 | 17.33 | 17.28 | 0.23 | 0.20 | 1.37 | 1.35 |
| 5 | 17.24 | 0.19 | 1.35 | |||||
| 7 | 17.29 | 0.19 | 1.31 | |||||
| 10 | 17.26 | 0.20 | 1.36 | |||||
| KAS method | ||||||||
| EP | 2 | 54.37 | 17.16 | 17.17 | 0.39 | 0.38 | 1.45 | 1.47 |
| 5 | 17.29 | 0.39 | 1.45 | |||||
| 7 | 17.10 | 0.33 | 1.47 | |||||
| 10 | 17.13 | 0.43 | 1.51 | |||||
| EP/Zn-Al-CO3-HA | 2 | 59.94 | 18.77 | 18.72 | 0.18 | 0.15 | 1.40 | 1.42 |
| 5 | 18.71 | 0.14 | 1.38 | |||||
| 7 | 18.70 | 0.15 | 1.46 | |||||
| 10 | 18.70 | 0.15 | 1.46 | |||||
| EP/Mg-Al-CO3-HA | 2 | 59.05 | 18.52 | 18.41 | 0.20 | 0.16 | 1.40 | 1.38 |
| 5 | 18.37 | 0.15 | 1.38 | |||||
| 7 | 18.41 | 0.14 | 1.34 | |||||
| 10 | 18.36 | 0.15 | 1.39 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, Z.; Ganjali, M.R.; Zarghami Dehaghani, M.; Aghazadeh, M.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; Mohaddespour, A.; Inamuddin; Formela, K.; et al. Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers 2020, 12, 1157. https://doi.org/10.3390/polym12051157
Karami Z, Ganjali MR, Zarghami Dehaghani M, Aghazadeh M, Jouyandeh M, Esmaeili A, Habibzadeh S, Mohaddespour A, Inamuddin, Formela K, et al. Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers. 2020; 12(5):1157. https://doi.org/10.3390/polym12051157
Chicago/Turabian StyleKarami, Zohre, Mohammad Reza Ganjali, Maryam Zarghami Dehaghani, Mustafa Aghazadeh, Maryam Jouyandeh, Amin Esmaeili, Sajjad Habibzadeh, Ahmad Mohaddespour, Inamuddin, Krzysztof Formela, and et al. 2020. "Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides" Polymers 12, no. 5: 1157. https://doi.org/10.3390/polym12051157
APA StyleKarami, Z., Ganjali, M. R., Zarghami Dehaghani, M., Aghazadeh, M., Jouyandeh, M., Esmaeili, A., Habibzadeh, S., Mohaddespour, A., Inamuddin, Formela, K., Haponiuk, J. T., & Saeb, M. R. (2020). Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers, 12(5), 1157. https://doi.org/10.3390/polym12051157