Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrophilic Acrylic Pre-Polymers
2.3. Fabrication of Hydrophilic Acrylic PSA Films
2.4. Characterization of Hydrophilic Acrylic Pre-Polymers and Acrylic PSA Films
2.5. Morphological and Rheological Analysis of Hydrophilic Acrylic PSA Films
2.6. Characterization of Affinity for Hydrophilic Materials and Biocompatibility
3. Results
3.1. Characterization of Synthesized Hydrophilic Acrylic Pre-Polymers and Acrylic PSA Films
3.2. Morphological Analysis of Acrylic PSA Films
3.3. Rheological Analysis of Acrylic PSA Films
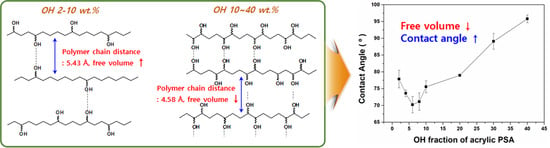
3.4. Characterization of Affinity for Hydrophilic Materials and Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fang, C.; Lin, Z. Effect of propyleneimine external cross-linker on the properties of acrylate latex pressure sensitive adhesives. Int. J. Adhes. Adhes. 2015, 61, 1–7. [Google Scholar] [CrossRef]
- Kim, D.H.; Seok, W.C.; Leem, J.T.; Han, Y.W.; Kang, J.H.; Song, H.J. Ordered orientation and compact molecule packing due to coplanar backbone structure of interlayer: Improvement in fill factor for photovoltaic device. Eur. Polym. J. 2019, 116, 330–335. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Li, N.; Bai, Y.; Zheng, X. Considerations of functional fluoropolymer structure in the design of acrylic–fluorine hybrid PSAs: Graft versus telechelic cooligomers. J. Appl. Polym. Sci. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Pang, B.; Ryu, C.M.; Kim, H., II. Effect of naphthyl curing agent having thermally stable structure on properties of UV-cured pressure sensitive adhesive. J. Ind. Eng. Chem. 2014, 20, 3195–3200. [Google Scholar] [CrossRef]
- Ko, K.Y.; Hwang, S.H. Monomer composition effects on thermal properties of transparent poly(methyl methacrylate-co-isobornyl methacrylate-co-cyclohexyl maleimide) terpolymers. J. Ind. Eng. Chem. 2018, 59, 50–55. [Google Scholar] [CrossRef]
- Baek, S.S.; Hwang, S.H. Preparation of biomass-based transparent pressure sensitive adhesives for optically clear adhesive and their adhesion performance. Eur. Polym. J. 2017, 92, 97–104. [Google Scholar] [CrossRef]
- Khan, I.; Poh, B.T. Natural Rubber-Based Pressure-Sensitive Adhesives: A Review. J. Polym. Environ. 2011, 19, 793–811. [Google Scholar] [CrossRef]
- Antosik, A.K.; Bednarczyk, P.; Czech, Z. Aging of silicone pressure-sensitive adhesives. Polym. Bull. 2018, 75, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.S.; Jang, S.J.; Hwang, S.H. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives: Effects of substituent structure of acrylate monomer. Int. J. Adhes. Adhes. 2016, 64, 72–77. [Google Scholar] [CrossRef]
- Beak, S.S.; Hong, S.; Hwang, S.H. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives containing biomass-derived menthol moiety. Polymer 2016, 40, 678–683. [Google Scholar] [CrossRef]
- Yang, J.; Keijsers, J.; Van Heek, M.; Stuiver, A.; Cohen Stuart, M.A.; Kamperman, M. The effect of molecular composition and crosslinking on adhesion of a bio-inspired adhesive. Polym. Chem. 2015, 6, 3121–3130. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z. Fluorinated poly (isobornyl methacrylate-co-butyl acrylate) core-shell latex nanoparticles: Synthesis, morphology and wettability of films. Polymer 2013, 54, 3047–3054. [Google Scholar] [CrossRef]
- Yang, D.; Wan, X.; Quan, P.; Liu, C.; Fang, L. The role of carboxyl group of pressure sensitive adhesive in controlled release of propranolol in transdermal patch: Quantitative determination of ionic interaction and molecular mechanism characterization. Eur. J. Pharm. Sci. 2018, 115, 330–338. [Google Scholar] [CrossRef]
- Zhong, J.; Ji, H.; Duan, J.; Tu, H.; Zhang, A. Coating morphology and surface composition of acrylic terpolymers with pendant catechol, OEG and perfluoroalkyl groups in varying ratio and the effect on protein adsorption. Colloids Surf. B Biointerfaces 2016, 140, 254–261. [Google Scholar] [CrossRef]
- Lee, S.B.; González-Cabezas, C.; Kim, K.M.; Kim, K.N.; Kuroda, K. Catechol-Functionalized Synthetic Polymer as a Dental Adhesive to Contaminated Dentin Surface for a Composite Restoration. Biomacromolecules 2015, 16, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Weng, F.; Li, J.; Lai, L.; Yu, W.; Severtson, S.J.; Wang, W.J. Influence of Phase Separation on Performance of Graft Acrylic Pressure-Sensitive Adhesives with Various Copolyester Side Chains. ACS Omega 2018, 3, 6945–6954. [Google Scholar] [CrossRef]
- Lee, J.G.; Shim, G.S.; Park, J.W.; Kim, H.J.; Han, K.Y. Kinetic and mechanical properties of dual curable adhesives for display bonding process. Int. J. Adhes. Adhes. 2016, 70, 249–259. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, S.J.; Lee, T.H.; Kim, H.J. Characterization of an acrylic polymer under hygrothermal aging as an optically clear adhesive for touch screen panels. Int. J. Adhes. Adhes. 2015, 63, 137–144. [Google Scholar] [CrossRef]
- Wang, H.; Pastorin, G.; Lee, C. Toward Self-Powered Wearable Adhesive Skin Patch with Bendable Microneedle Array for Transdermal Drug Delivery. Adv. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Quan, P.; Li, S.; Zhao, Y.; Fang, L. A systemic evaluation of drug in acrylic pressure sensitive adhesive patch in vitro and in vivo: The roles of intermolecular interaction and adhesive mobility variation in drug controlled release. J. Control. Release 2017, 252, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Malvey, S.; Rao, J.V.; Arumugam, K.M. Transdermal Drug Delivery Systems: A Mini Review. Pharma Innov. J. 2019, 8, 181–197. [Google Scholar]
- Subedi, R.K.; Oh, S.Y.; Chun, M.K.; Choi, H.K. Recent advances in transdermal drug delivery. Arch. Pharm. Res. 2010, 33, 339–351. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Y.; Zhang, C.; Li, Z. Optimization of SIS-based hot-melt pressure-sensitive adhesives for transdermal delivery of hydrophilic drugs. Int. J. Adhes. Adhes. 2016, 68, 256–262. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, R.; Zhang, C.; Wang, Q. SISO-based hot-melt pressure-sensitive adhesives for transdermal delivery of hydrophilic drugs. Int. J. Adhes. Adhes. 2017, 74, 86–91. [Google Scholar] [CrossRef]
- Schier, J.E.S.; Hutchinson, R.A. The influence of hydrogen bonding on radical chain-growth parameters for butyl methacrylate/2-hydroxyethyl acrylate solution copolymerization. Polym. Chem. 2016, 7, 4567–4574. [Google Scholar] [CrossRef] [Green Version]
- Schier, J.E.S.; Zhang, M.; Grady, M.C.; Hutchinson, R.A. Modeling of Semibatch Solution Radical Copolymerization of Butyl Methacrylate and 2-Hydroxyethyl Acrylate. Macromol. React. Eng. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Bian, K.; Cunningham, M.F. Nitroxide-mediated living radical polymerization of 2-hydroxyethyl acrylate and the synthesis of amphiphilic block copolymers. Macromolecules 2005, 38, 695–701. [Google Scholar] [CrossRef]
- Schier, J.E.S.; Cohen-Sacal, D.; Larsen, O.R.; Hutchinson, R.A. The effect of hydrogen bonding on radical semi-batch copolymerization of butyl acrylate and 2-hydroxyethyl acrylate. Polymers 2017, 9, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.S.; Wu, Y.C.; Kuo, S.W. Thymine-and adenine-functionalized polystyrene form self-assembled structures through multiple complementary hydrogen bonds. Polymers 2014, 6, 1827–1845. [Google Scholar] [CrossRef] [Green Version]
- Morita, S. Hydrogen-bonds structure in poly (2-hydroxyethyl methacrylate) studied by temperature-dependent infrared spectroscopy. Front. Chem. 2014, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Hwang, D.K.; Lee, H.S. Thermal properties in strong hydrogen bonding systems composed of poly(Vinyl alcohol), polyethyleneimine, and graphene oxide. Carbon Lett. 2014, 15, 282–289. [Google Scholar] [CrossRef]
- Kuo, S.W.; Xu, H.; Huang, C.F.; Chang, F.C. Significant glass-transition-temperature increase through hydrogen-bonded copolymers. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2313–2323. [Google Scholar] [CrossRef]
- Baek, S.S.; Hwang, S.H. Eco-friendly UV-curable pressure sensitive adhesives containing acryloyl derivatives of monosaccharides and their adhesive performances. Int. J. Adhes. Adhes. 2016, 70, 110–116. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Kabatc, J.; Swiderska, J. Thermal stability of poly (2-ethylhexyl acrylates) used as plasticizers for medical application. Polym. Bull. 2013, 70, 1911–1918. [Google Scholar] [CrossRef] [Green Version]
- Ozlem, S.; Aslan-Gürel, E.; Rossi, R.M.; Hacaloglu, J. Thermal degradation of poly (isobornyl acrylate) and its copolymer with poly (methyl methacrylate) via pyrolysis mass spectrometry. J. Anal. Appl. Pyrolysis 2013, 100, 17–25. [Google Scholar] [CrossRef]
- Vargün, E.; Usanmaz, A. Polymerization of 2-hydroxyethyl acrylate in bulk and solution by chemical initiator and by ATRP method. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3957–3965. [Google Scholar] [CrossRef]
- Hong, J.L.; Zhang, X.H.; Wei, R.J.; Wang, Q.; Fan, Z.Q.; Qi, G.R. Inhibitory effect of hydrogen bonding on thermal decomposition of the nanocrystalline cellulose/poly (propylene carbonate) nanocomposite. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Kang, H.G.; Kim, M. X-ray Photoelectron Spectroscopy to Characterize Polymer Thin Films. Polym. Sci. Technol. 2016, 27, 245–253. [Google Scholar]
- Fan, X.; Jia, X.; Liu, Y.; Zhang, B.; Li, C.; Liu, Y.; Zhang, H.; Zhang, Q. Tunable wettability of hierarchical structured coatings derived from one-step synthesized raspberry-like poly(styrene-acrylic acid) particles. Polym. Chem. 2015, 6, 703–713. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Kim, J.H.; Gadisa, A.; Schaefer, C.; Yao, H.; Gautam, B.R.; Balar, N.; Ghasemi, M.; Constantinou, I.; So, F.; O’Connor, B.T.; et al. Strong polymer molecular weight-dependent material interactions: Impact on the formation of the polymer/fullerene bulk heterojunction morphology. J. Mater. Chem. A 2017, 5, 13176–13188. [Google Scholar] [CrossRef]
- Jang, S.J.; Baek, S.S.; Kim, J.Y.; Hwang, S.H. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives for touch screen panel. J. Adhes. Sci. Technol. 2014, 28, 1990–2000. [Google Scholar] [CrossRef]
- Kowalski, A.; Czech, Z. The effects of substrate surface properties on tack performance of acrylic Pressure-Sensitive Adhesives (PSAs). Int. J. Adhes. Adhes. 2015, 60, 9–15. [Google Scholar] [CrossRef]
- Baek, S.S.; Jang, S.J.; Lee, J.H.; Kho, D.H.; Lee, S.H.; Hwang, S.H. Preparation of Acrylic Sensitive Adhesives for Optical Applications and Their Adhesion Performance. Polymer 2013, 38, 199–204. [Google Scholar]
- Hwang, S.O.; Lee, A.S.; Lee, J.Y.; Park, S.H.; Jung, K.I.; Jung, H.W.; Lee, J.H. Mechanical properties of ladder-like polysilsesquioxane-based hard coating films containing different organic functional groups. Prog. Org. Coat. 2018, 121, 105–111. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, M.; Drummey, K.J.; Talley, S.J.; Anderson, L.J.; Moore, R.B.; Long, T.E. Ureido cytosine and cytosine-containing acrylic copolymers. Polym. Chem. 2016, 7, 6671–6681. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.C.; Wang, J.H.; Chuang, W.T.; Liao, Z.S.; Huang, J.J.; Huang, S.Y.; Fan, W.L.; Lee, D.J. Dynamic supramolecular self-assembly hydrogen bonding-induced contraction and extension of functional polymers. Polym. Chem. 2017, 8, 3294–3299. [Google Scholar] [CrossRef]
- Luo, L.; Yao, J.; Wang, X.; Li, K.; Huang, J.; Li, B.; Wang, H.; Feng, Y.; Liu, X. The evolution of macromolecular packing and sudden crystallization in rigid-rod polyimide via effect of multiple H-bonding on charge transfer (CT) interactions. Polymer 2014, 55, 4258–4269. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Gilberto, O. Chierice Characterization of Polyurethane Resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Zang, X.L.; Wang, G.X.; Wang, P.L.; Lu, B.; Ji, J.H. Poly (butylene 2,5-furandicarboxylate-ε-caprolactone): A new bio-based elastomer with high strength and biodegradability. Express Polym. Lett. 2017, 11, 611–621. [Google Scholar] [CrossRef]
- Yano, H.; Kudo, K.; Marumo, K.; Okuzaki, H. Fully soluble self-doped poly (3,4-ethylenedioxythiophene) with an electrical conductivity greater than 1000 S cm−1. Sci. Adv. 2019, 5, eaav9492. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.Y.; Roper, T.M.; Jönsson, E.S.; Guymon, C.A.; Hoyle, C.E. Influence of hydrogen bonding on photopolymerization rate of hydroxyalkyl acrylates. Macromolecules 2004, 37, 3659–3665. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards Dynamic but Supertough Healable Polymers through Biomimetic Hierarchical Hydrogen-Bonding Interactions. Angew. Chem.-Int. Ed. 2018, 57, 13838–13842. [Google Scholar] [CrossRef]










| Acrylic Syrups | EHA a | IBOA b | HEA c | Characterization | ||||
|---|---|---|---|---|---|---|---|---|
| Mn (g/mol) | Mw (g/mol) | PDI | Tgd (°C) | Tde (°C) | ||||
| Acryl-2 | 50 | 48 | 2 | 441,000 | 568,000 | 1.29 | −9.50 | 298 |
| Acryl-4 | 46 | 4 | 335,000 | 478,000 | 1.43 | −9.15 | 303 | |
| Acryl-6 | 44 | 6 | 328,000 | 504,000 | 1.53 | −9.11 | 306 | |
| Acryl-8 | 42 | 8 | 331,000 | 518,000 | 1.56 | −6.86 | 303 | |
| Acryl-10 | 40 | 10 | 312,000 | 645,000 | 2.07 | −9.88 | 302 | |
| Acryl-20 | 30 | 20 | 382,000 | 763,000 | 1.99 | −10.98 | 301 | |
| Acryl-30 | 20 | 30 | 374,000 | 788,000 | 2.11 | −12.77 | 289 | |
| Acryl-40 | 10 | 40 | 616,000 | 880,000 | 1.43 | −13.84 | 286 | |
| Sample | Binding Energy (eV) | ||||
|---|---|---|---|---|---|
| EC-C | Intensity | EC-O | Intensity | EC-C/EC-O | |
| Acryl-2 | 283.78 | 67,200 | 285.28 | 13,200 | 5.09 |
| Acryl-6 | 283.78 | 66,900 | 285.28 | 13,600 | 4.92 |
| Acryl-10 | 283.98 | 67,300 | 285.38 | 13,900 | 4.84 |
| Acryl-20 | 283.98 | 66,900 | 285.48 | 14,400 | 4.65 |
| Acryl-30 | 283.98 | 66,800 | 285.58 | 15,700 | 4.25 |
| Acryl-40 | 284.08 | 67,000 | 285.58 | 15,500 | 4.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, W.C.; Leem, J.T.; Kang, J.H.; Kim, Y.J.; Lee, S.; Song, H.J. Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio. Polymers 2020, 12, 1504. https://doi.org/10.3390/polym12071504
Seok WC, Leem JT, Kang JH, Kim YJ, Lee S, Song HJ. Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio. Polymers. 2020; 12(7):1504. https://doi.org/10.3390/polym12071504
Chicago/Turabian StyleSeok, Woong Cheol, Jong Tae Leem, Ju Hui Kang, Young Jun Kim, Sangkug Lee, and Ho Jun Song. 2020. "Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio" Polymers 12, no. 7: 1504. https://doi.org/10.3390/polym12071504
APA StyleSeok, W. C., Leem, J. T., Kang, J. H., Kim, Y. J., Lee, S., & Song, H. J. (2020). Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio. Polymers, 12(7), 1504. https://doi.org/10.3390/polym12071504