The Buffer Effect of Different Wood Species and the Influence of Oak on Panel Composites Binders
Abstract
:1. Introduction
2. Materials and Methods
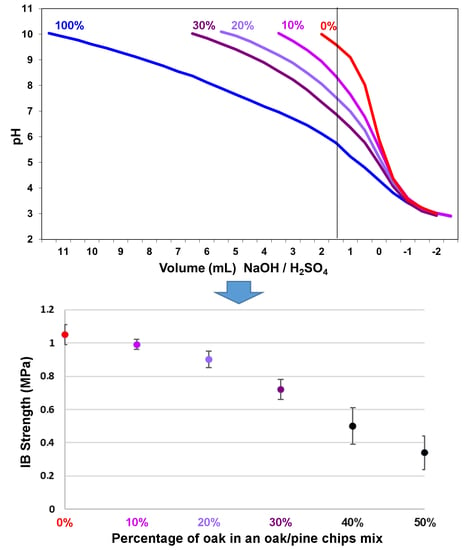
- v1 = volume in ml of NaOH 0.05 N added up to reach a pH of 10.
- v2 = volume in ml of H2SO4 0.05 N added up to reach a pH of 3.
2.1. MUF Resin Preparation
2.2. UF Resin Preparation
2.3. Particleboard Testing
2.4. Buffer Capacity of Defibered Wood Mixtures and Glued with UF and MUF Adhesives
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saravia-Cortez, A.M.; Herva, M.; García-Diéguez, C.; Roca, E. Assessing environmental sustainability of particleboard production process by ecological footprint. J. Clean. Prod. 2013, 52, 301–308. [Google Scholar] [CrossRef]
- Gonçalves, C.; Paiva, N.T.; Ferra, J.M.; Martins, J.; Magalhães, F.; Barros-Timmons, A.; Carvalho, L. Utilization and characterization of amino resins for the production of wood-based panels with emphasis on particleboards (PB) and medium density fibreboards (MDF). A review. Holzforschung 2018, 72, 653–671. [Google Scholar] [CrossRef]
- Bockel, S.; Mayer, I.; Konnerth, J.; Niemz, P.; Swaboda, C.; Beyer, M.; Harling, S.; Weiland, G.; Bieri, N.; Pichelin, F. Influence of wood extractives on two-component polyurethane adhesive for structural hardwood bonding. J. Adhes. 2018, 94, 829–845. [Google Scholar] [CrossRef]
- Hse, C.-Y. Wettability of Southern Pine Veneer by Phenol Formaldehyde Wood Adhesives. For. Prod. J. 1972, 22, 51–56. [Google Scholar]
- Mantanis, G.I.; Young, R.A. Wetting of wood. Wood Sci. Technol. 1997, 31, 339–353. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Factor Affecting Gel Time/Process-Ability of Urea Formaldehyde Resin Based Wood Adhesives. Open J. Polym. Chem. 2017, 7, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, A.; Hale, M.D.; Spear, M.J. The effect of pH on surface activation of wood polymer composites (WPCs) with hydrogen peroxide for improved adhesion. Int. J. Adhes. Adhes. 2018, 85, 44–57. [Google Scholar] [CrossRef]
- Johns, W.E.; Niazi, K.A. Effect of pH and buffering capacity of wood on the relation time of urea formaldehyde resin. Wood Fiber Sci. 1980, 12, 255–263. [Google Scholar]
- Wang, X.; Huang, Z.; Cooper, P.; Wang, X.-M.; Zhang, Y.; Casilla, R. The Ability of Wood to Buffer Highly Acidic and Alkaline Adhesives. Wood Fiber Sci. 2010, 42, 398–405. [Google Scholar]
- Wilson, J.B. Life-cycle inventory of particleboard in terms of resources, emissions, energy and carbon. Wood Fiber Sci. 2010, 42, 90–106. [Google Scholar]
- Parikka, M. Global biomass fuel resources. Biomass Bioenergy 2004, 27, 613–620. [Google Scholar] [CrossRef]
- Albert, L.; Németh, Z.I.; Halász, G.; Koloszár, J.; Varga, S.; Takács, L. Radial variation of pH and buffer capacity in the red-heartwooded beech (Fagus silvatica L.) wood. Holz als Roh-und Werkst. 1999, 57, 75–76. [Google Scholar] [CrossRef]
- Pedieu, R.; Riedl, B.; Pichette, A. Measurement of wood and bark particles acidity and their impact on the curing of urea formaldehyde resin during the hot pressing of mixed panels. Holz als Roh-und Werkst. 2008, 66, 113–117. [Google Scholar] [CrossRef]
- Passialis, C.; Voulgaridis, E.; Adamopoulos, S.; Matsouka, M. Extractives, acidity, buffering capacity, ash and inorganic elements of black locust wood and bark of different clones and origin. Holz als Roh-und Werkst. 2008, 66, 395–400. [Google Scholar] [CrossRef]
- Irle, M.A.; Barbu, M.C.; Reh, R.; Bergland, L.; Rowell, R.M. Wood Composites. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 321–412. [Google Scholar]
- Andre, N.; Young, T.M.; Rials, T.G. On-Line Monitoring of the Buffer Capacity of Particleboard Furnish by Near-Infrared Spectroscopy. Appl. Spectrosc. 2006, 60, 1204–1209. [Google Scholar] [CrossRef]
- Blomquist, R.F.; Christiansen, A.W.; Gillespie, R.H.; Myers, G.E. Adhesive bonding of wood and other structural materials. In Clark, C. Heritage Memorial Workshop on Wood 1981: Madison, Wis.; Educational Modules for Materials Science and Engineering (EMMSE) Project Materials Research Laboratory, the Pennsylvania State University: State College, PA, USA, 1983; pp. 12–110. [Google Scholar]
- DeMarco, F.A.; Smith, E.A. Determination of Relative Acidity of Wood and Adhesive Joints. Ind. Eng. Chem. Anal. Ed. 1946, 18, 775–777. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, S.Y.; Deng, J.; Riedl, B.; Cloutier, A. Medium-density fiberboard performance as affected by wood fiber acidity, bulk density, and size distribution. Wood Sci. Technol. 2006, 40, 637–646. [Google Scholar] [CrossRef]
- Maloney, T.M. Modern Particleboard and Dry-Process Fiber Board Manufacturing; Miller Freeman Publication: San Francisco, CA, USA, 1993. [Google Scholar]
- Zanetti, M.; Pizzi, A. Upgrading of MUF polycondensation resins by buffering additives. II. Hexamine sulfate mechanisms and alternate buffers. J. Appl. Polym. Sci. 2003, 90, 215–226. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Kamoun, C. Upgrading of MUF particleboard adhesives and decrease of melamine content by buffer and additives. Holz als Roh-und Werkst. 2003, 61, 55–65. [Google Scholar] [CrossRef]
- Stefke, B.; Dunky, M. Catalytic influence of wood on the hardening behavior of formaldehyde-based resin adhesives used for wood-based panels. J. Adhes. Sci. Technol. 2006, 20, 761–785. [Google Scholar] [CrossRef]
- Park, B.; Kim, Y.S.; Riedl, B. Effect of wood-fiber characteristics on medium density. J. Korean Wood Sci. Technol. 2001, 29, 27–35. [Google Scholar]
- Xing, C.; Zhang, S.Y.; Deng, J. Effect of wood acidity and catalyst on UF resin gel time. Holzforschung 2004, 58, 408–412. [Google Scholar] [CrossRef]
- Wang, X.M.; Casilla, R.; Zhang, Y.; Cooper, P.; Huang, Z.; Wang, X.A. Effect of Extreme pH on Bond Durability of Selected Structural Wood Adhesives. Wood Fiber Sci. 2016, 48, 245–259. [Google Scholar]
- Wang, X.; Huang, Z.; Cooper, P.; Wang, X.M.; Zhang, Y.; Casilla, R. Effects of pH on lap-shear strength for aspen veneer. Wood Fiber Sci. 2013, 45, 294–302. [Google Scholar]
- Huang, Z.; Cooper, P.; Wang, X.; Wang, X.M.; Zhang, Y.; Casilla, R. Effects of conditioning exposure on the PH distribution near adhesive-wood bond lines. Wood Fiber Sci. 2010, 42, 219–228. [Google Scholar]
- Riedl, B.; He, G. Curing kinetics of phenol formaldehyde resin and wood-resin interactions in the presence of wood substrates. Wood Sci. Technol. 2004, 38, 69–81. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, S.; Yu, W.; Deng, Y. Temperature-dependent mechanical properties of wood-adhesive bondline evaluated by nanoindentation. J. Adhes. 2017, 93, 640–656. [Google Scholar] [CrossRef]
- Xing, C.; Deng, J.; Zhang, S.Y.; Riedl, B.; Cloutier, A. Differential scanning calorimetry characterization of urea-formaldehyde resin curing behavior as affected by less desirable wood material and catalyst content. J. Appl. Polym. Sci. 2005, 98, 2027–2032. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.-M.; Wan, H.; Liu, Y. Curing characteristics of urea–formaldehyde resin in the presence of various amounts of wood extracts and catalysts. J. Appl. Polym. Sci. 2008, 107, 1555–1562. [Google Scholar] [CrossRef]
- Sandermann, W.; Rothkamm, M. The determination of pH values of woods and their practical importance. Holz Roh Werkst. 1959, 17, 433–440. [Google Scholar] [CrossRef]
- Kamoun, C.; Pizzi, A.; Zanetti, M. Upgrading of MUF resins by buffering additives–Part 1: Hexamine sulphate effect and its limits. J. Appl. Polym. Sci. 2003, 90, 203–214. [Google Scholar] [CrossRef]
- Pizzi, A. Urea and melamine aminoresin adhesives. In Handbook of Addhesive Technology, 3rd ed.; Pizzi, A., Mittal, K., Eds.; CRC Press: New York, NY, USA, 2017; pp. 283–320. [Google Scholar]
- Meier, E. The Wood Database. Available online: https://www.wood-database.com/ (accessed on 19 May 2020).




| Wood Chips Oak:Pine | IB Strength (MPa) | Average Density Dry (kg/m3) |
|---|---|---|
| 0:100 | 1.05 ± 0.06 | 0.710 ± 0.0094 |
| 10:90 | 0.99 ± 0.03 | 0.705 ± 0.0062 |
| 20:80 | 0.90 ± 0.05 | 0.700 ± 0.0102 |
| 30:70 | 0.72 ± 0.06 | 0.680 ± 0.0058 |
| 40:60 | 0.50 ± 0.11 | 0.670 ± 0.0113 |
| 50:50 | 0.34 ± 0.10 | 0.650 ± 0.0098 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policardi, F.; Thebault, M. The Buffer Effect of Different Wood Species and the Influence of Oak on Panel Composites Binders. Polymers 2020, 12, 1540. https://doi.org/10.3390/polym12071540
Policardi F, Thebault M. The Buffer Effect of Different Wood Species and the Influence of Oak on Panel Composites Binders. Polymers. 2020; 12(7):1540. https://doi.org/10.3390/polym12071540
Chicago/Turabian StylePolicardi, Franco, and Marion Thebault. 2020. "The Buffer Effect of Different Wood Species and the Influence of Oak on Panel Composites Binders" Polymers 12, no. 7: 1540. https://doi.org/10.3390/polym12071540
APA StylePolicardi, F., & Thebault, M. (2020). The Buffer Effect of Different Wood Species and the Influence of Oak on Panel Composites Binders. Polymers, 12(7), 1540. https://doi.org/10.3390/polym12071540