Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review
Abstract
:1. Introduction
2. Cutaneous Wounds
3. Skin Tissue Engineering and Regenerative Medicine
4. Techniques of Skin Tissue Engineering
5. Components of Skin Tissue Engineering and Regenerative Medicine
5.1. Growth Factors
5.2. Cells and Cellular Skin Substitutes
5.3. Stem Cells
5.4. Biomaterials
6. Challenges and Limitations in Stem Cell Therapeutics for Wound Healing
7. Combinational Therapy Using Both Biomaterials and Stem Cell in Wound Healing and Regeneration Treatment
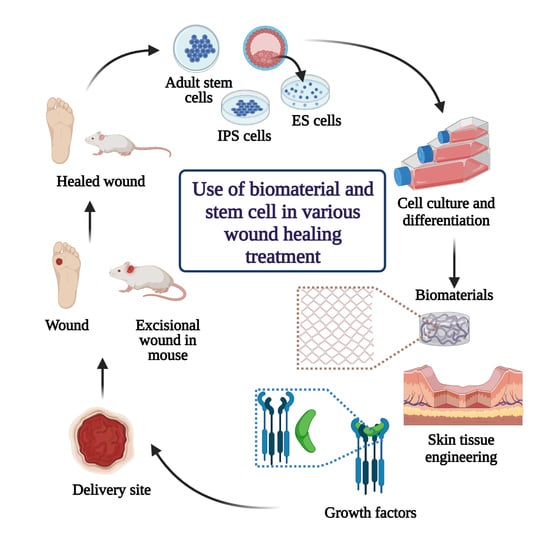
8. Role of Biomaterials and Stem Cells in Skin Tissue Engineering for Wound Healing and Regeneration Treatment
9. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current advancements and strategies in tissue engineering for wound healing: A comprehensive review. Adv. Wound Care (New Rochelle) 2017, 6, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Rezaie, F.; Momeni-Moghaddam, M.; Naderi-Meshkin, H. Regeneration and repair of skin wounds: Various strategies for treatment. Int. J. Low. Extrem. Wounds 2019, 18, 247–261. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Chapman, M.A. Regenerative medicine: Charting a new course in wound healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar] [CrossRef]
- Kim, H.; Hyun, M.R.; Kim, S.W. The effect of adipose-derived stem cells on wound healing: Comparison of methods of application. Stem Cells Int. 2019, 2745640. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Holmes, J.H.T.; Nam, A.J.; Fisher, J.P. Current and future perspectives on skin tissue engineering: Key features of biomedical research, translational assessment, and clinical application. Adv. Healthc. Mater. 2019, 8, 1801471. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound healing concepts in clinical practice of OMFS. J. Maxillofac. Oral Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, M.B.; Barroso, W.A.; Silva, N.N.; Silva, S.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M.O.R. From inflammation to current and alternative therapies involved in wound healing. Int. J. Inflamm. 2017, 3406215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. 2), 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrantonaki, E.; Wlaschek, M.; Scharffetter-Kochanek, K. Pathogenesis of wound healing disorders in the elderly. J. Dtsch. Dermatol. Ges. 2017, 15, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burn. Trauma 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Dhasmana, A.; Singh, S.; Kadian, S.; Singh, L. Skin tissue engineering: Principles and advances. J. Dermatol. Skin 2018, 1, 3–6. [Google Scholar]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Methodologies in creating skin substitutes. Cell. Mol. Life Sci. 2016, 73, 3453–3472. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L. Tissue engineering and regenerative medicine: New trends and directions-A year in review. Tissue Eng. Part B Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef]
- Nakayama, C.; Fujita, Y.; Matsumura, W.; Ujiie, I.; Takashima, S.; Shinkuma, S.; Nomura, T.; Abe, R.; Shimizu, H. The development of induced pluripotent stem cell-derived mesenchymal stem/stromal cells from normal human and RDEB epidermal keratinocytes. J. Dermatol. Sci. 2018, 91, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, B.N.; Hahn, J.M.; McFarland, K.L.; DeBruler, D.M.; Supp, D.M.; Powell, H.M. Inflammatory response and biomechanical properties of coaxial scaffolds for engineered skin in vitro and post-grafting. Acta Biomater. 2018, 80, 247–257. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A Review. Adv. Mater. Sci. Eng. 2019, 3429527. [Google Scholar] [CrossRef] [Green Version]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef] [Green Version]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 151–174. [Google Scholar]
- Manavitehrani, I.; Le, T.Y.L.; Daly, S.; Wang, Y.; Maitz, P.K.; Schindeler, A.; Dehghani, F. Formation of porous biodegradable scaffolds based on poly(propylene carbonate) using gas foaming technology. Mater. Sci. Eng. C 2019, 96, 824–830. [Google Scholar] [CrossRef]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Biswas, D.P.; Tran, P.A.; Tallon, C.; O′Connor, A.J. Combining mechanical foaming and thermally induced phase separation to generate chitosan scaffolds for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthetic. Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Zhang, B.; Cristescu, R.; Chrisey, D.B.; Narayan, R.J. Solvent-based extrusion 3D Printing for the fabrication of tissue engineering scaffolds. Int. J. Bioprinting 2020, 6, 211. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Farzan, A.; Borandeh, S.; Zanjanizadeh Ezazi, N.; Lipponen, S.; Santos, H.A.; Seppala, J. 3D scaffolding of fast photocurable polyurethane for soft tissue engineering by stereolithography: Influence of materials and geometry on growth of fibroblast cells. Eur. Polym. J. 2020, 139, 109988. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singaravelu, S.; Muthukumar, T.; Thyagarajan, S.; Perumal, P.T.; Sivagnanam, U.T. Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Mater. Sci. Eng. C 2017, 72, 359–370. [Google Scholar] [CrossRef]
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.-K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, K.M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef]
- Li, M.; Qiu, L.; Hu, W.; Deng, X.; Xu, H.; Cao, Y.; Xiao, Z.; Peng, L.; Johnson, S.; Alexey, L.; et al. Genetically-modified bone mesenchymal stem cells with TGF-beta3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp. Cell Res. 2018, 367, 24–29. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Park, M.; Ban, E.; Lee, S.H.; Kim, A. Improved diabetic wound healing by EGF encapsulation in gelatin-alginate coacervates. Pharmaceutics 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; An, N.; Zhang, H.; Zhang, Q.; Zhang, K.; Hu, X.; Wu, Y.; Wu, F.; Xiao, J.; Zhang, H.; et al. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J. Drug. Deliv. Technol. 2020, 55, 101405. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef]
- Pang, C.; Ibrahim, A.; Bulstrode, N.W.; Ferretti, P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int. Wound J. 2017, 14, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.Y.; Wu, J.; Zhu, J.J.; Xiao, Z.C.; He, C.C.; Shi, H.X.; Li, X.K.; Yang, S.L.; Xiao, J. Research advances in tissue engineering materials for sustained release of growth factors. Biomed. Res. Int. 2015, 808202. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Kallis, P.J.; Friedman, A.J.; Lev-Tov, H. A guide to tissue-engineered skin substitutes. J. Drugs Dermatol. 2018, 17, 57–64. [Google Scholar]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts-A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18–32. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Yeung, J. Current status and future of skin substitutes for chronic wound healing. J. Cutan. Med. Surg. 2017, 21, 23–30. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Bioengineered skin substitutes: Advances and future trends. Appl. Sci. 2021, 11, 1493. [Google Scholar] [CrossRef]
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The basics of integra dermal regeneration template and its expanding clinical applications. Semin. Plast. Surg. 2019, 33, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem cells in wound healing: The future of regenerative medicine? A mini-review. Gerontology 2016, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem cells and engineered scaffolds for regenerative wound healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosaric, N.; Kiwanuka, H.; Gurtner, G.C. Stem cell therapies for wound healing. Expert. Opin. Biol. Ther. 2019, 19, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Das, H. Advances of stem cell therapeutics in cutaneouswound healing and regeneration. Mediat. Inflamm. 2017, 5217967. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Sun, Y.; Zhang, J.; Zhu, Q.; Yang, Y.; Niu, X.; Deng, Z.; Li, Q.; Wang, Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell. Res. Ther. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Raghuram, A.C.; Yu, R.P.; Lo, A.Y.; Sung, C.J.; Bircan, M.; Thompson, H.J.; Wong, A.K. Role of stem cell therapies in treating chronic wounds: A systematic review. World J. Stem Cells 2020, 12, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.D.F.; Cunha, P.D.S.; Carregal, V.M.; Silva, P.D.C.D.; Miranda, M.C.D.; Kunrath-Lima, M.; de Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frezard, F.; et al. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate AKT pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem Cells Int. 2017, 9841035. [Google Scholar] [CrossRef]
- Li, D.J.; Shen, C.A.; Sun, T.J.; Zhang, L.; Deng, H.P.; Chai, J.K. Mesenchymal stem cells promote incision wound repair in a mouse model. Trop. J. Pharm. Res. 2017, 16, 1317–1323. [Google Scholar] [CrossRef] [Green Version]
- Kocan, B.; Maziarz, A.; Tabarkiewicz, J.; Ochiya, T.; Banas-Zabczyk, A. Trophic activity and phenotype of adipose tissue-derived mesenchymal stem cells as a background of their regenerative potential. Stem Cells Int. 2017, 1653254. [Google Scholar] [CrossRef]
- Yu, J.; Wang, M.Y.; Tai, H.C.; Cheng, N.C. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018, 77, 191–200. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef]
- Jung, J.A.; Yoon, Y.D.; Lee, H.W.; Kang, S.R.; Han, S.K. Comparison of human umbilical cord blood-derived mesenchymal stem cells with healthy fibroblasts on wound-healing activity of diabetic fibroblasts. Int. Wound J. 2018, 15, 133–139. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Wu, H.; Huang, Y.; Weitz, D.A. Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells. Biomaterials 2020, 248, 120014. [Google Scholar] [CrossRef]
- Huo, J.; Sun, S.; Geng, Z.; Sheng, W.; Chen, R.; Ma, K.; Sun, X.; Fu, X. Bone marrow-derived mesenchymal stem cells promoted cutaneous wound healing by regulating keratinocyte migration via beta2-adrenergic receptor signaling. Mol. Pharm. 2018, 15, 2513–2527. [Google Scholar] [CrossRef]
- Clayton, Z.E.; Tan, R.P.; Miravet, M.M.; Lennartsson, K.; Cooke, J.P.; Bursill, C.A.; Wise, S.G.; Patel, S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018, 38, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Su, Y.; Huang, C.; Yin, Y.; Chu, A.; Knupp, A.; Tang, Y. NANOG and LIN28 dramatically improve human cell reprogramming by modulating LIN41 and canonical WNT activities. Biol. Open 2019, 8, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.R.; Wang, C.T.; Cheng, J.T.; Kao, G.S.; Chiang, Y.C.; Wang, C.J. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant. 2016, 25, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P. Introduction to biomaterials for wound healing. In Wound Healing Biomaterials; Agren, M.S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–38. [Google Scholar]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikholeslam, M.; Wright, M.E.E.; Jeschke, M.G.; Amini-Nik, S. Biomaterials for skin substitutes. Adv. Healthc. Mater. 2018, 7, 1700897. [Google Scholar] [CrossRef]
- Akturk, O.; Kismet, K.; Yasti, A.C.; Kuru, S.; Duymus, M.E.; Kaya, F.; Caydere, M.; Hucumenoglu, S.; Keskin, D. Collagen/gold nanoparticle nanocomposites: A potential skin wound healing biomaterial. J. Biomater. Appl. 2016, 31, 283–301. [Google Scholar] [CrossRef]
- Amirrah, N.I.; Mohd Razip Wee, M.F.; Tabata, Y.; Bt Hj Idrus, R.; Nordin, A.; Fauzi, M.B. Antibacterial-integrated collagen wound dressing for diabetes-related foot ulcers: An evidence-based review of clinical studies. Polymers 2020, 12, 2168. [Google Scholar] [CrossRef]
- Arif, M.M.A.; Fauzi, M.B.; Nordin, A.; Hiraoka, Y.; Tabata, Y.; Yunus, M.H.M. Fabrication of bio-based gelatin sponge for potential use as a functional acellular skin substitute. Polymers 2020, 12, 2678. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K.; Kiran, V.; Hasnain, M.S.; Nayak, A.K. Chapter 13-Alginate-based hydrogel systems for drug releasing in wound healing. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 323–358. [Google Scholar]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Suesca, E.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C.; Fontanilla, M.R. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 333–341. [Google Scholar] [CrossRef]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Gsib, O.; Egles, C.; Bencherif, S. Fibrin: An underrated biopolymer for skin tissue engineering. J. Mol. Biol. Biotecnol. 2017, 2, 3. [Google Scholar]
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk fibroin-based biomaterials for biomedical applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Foong, C.Y.; Hamzah, M.S.A.; Razak, S.I.A.; Saidin, S.; Nayan, N.H.M. Influence of poly(lactic acid) layer on the physical and antibacterial properties of dry bacterial cellulose sheet for potential acute wound healing materials. Fibers Polym. 2018, 19, 263–271. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Datta, S.; Menon, G. Nanofibers from polyhydroxyalkanoates and their applications in tissue engineering. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 409–420. [Google Scholar]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Ferri, J.M.; Jorda, J.; Montanes, N.; Fenollar, O.; Balart, R. Manufacturing and characterization of poly(lactic acid) composites with hydroxyapatite. J. Thermoplast. Compos. Mater. 2017, 31, 865–881. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cell Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Cabello, J.C.; De Torre, I.G.; Cipriani, F.; Poocza, L. Elastin-like materials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 309–327. [Google Scholar]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2020, e2000619. [Google Scholar] [CrossRef]
- Naomi, R.; Fauzi, M.B. Cellulose/collagen dressings for diabetic foot ulcer: A review. Pharmaceutics 2020, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Grande, D.; Ramier, J.; Versace, D.L.; Renard, E.; Langlois, V. Design of functionalized biodegradable PHA-based electrospun scaffolds meant for tissue engineering applications. New Biotechnol. 2017, 37, 129–137. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Nikolaeva, E.D.; Vinogradova, O.N.; Volova, T.G. Experimental wound dressings of degradable PHA for skin defect repair. J. Mater. Sci. Mater. Med. 2016, 27, 165. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy Reddy, S.H.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Broder, M.S.; Quock, T.P.; Chang, E.; Reddy, S.R.; Agarwal-Hashmi, R.; Arai, S.; Villa, K.F. The cost of hematopoietic stem-cell transplantation in the United States. Am. Health Drug Benefits 2017, 10, 366–374. [Google Scholar]
- Ham, R.M.T.; Hovels, A.M.; Hoekman, J.; Frederix, G.W.J.; Leufkens, H.G.M.; Klungel, O.H.; Jedema, I.; Veld, S.A.J.; Nikolic, T.; Van Pel, M.; et al. What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy 2020, 22, 388–397. [Google Scholar] [PubMed]
- Masri, S.; Fauzi, M.B. Current insight of printability quality improvement strategies in natural-based bioinks for skin regeneration and wound healing. Polymers 2021, 13, 1011. [Google Scholar] [CrossRef]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X.; et al. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem Cell Res. Ther. 2018, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Alapure, B.V.; Lu, Y.; He, M.; Chu, C.C.; Peng, H.; Muhale, F.; Brewerton, Y.L.; Bunnell, B.; Hong, S. Accelerate healing of severe burn wounds by mouse bone marrow mesenchymal stem cell-seeded biodegradable hydrogel scaffold synthesized from arginine-based poly(ester amide) and chitosan. Stem Cells Dev. 2018, 27, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, J.; Gao, X.; Fereydooni, A.; Dash, C.B.; Luo, J.; Lee, R.S.; Taniguchi, R.; Hsia, H.; Qyang, Y.; Dardik, A. hiPSC-SMC incraeses angiogenesis and accelarate diabetic wound healing. Regen. Med. 2020, 15, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1alpha gene-activated collagen scaffold restores pro-angiogenic wound healing features in human diabetic adipose-derived stem cells. Biomedicines 2021, 9, 160. [Google Scholar] [CrossRef]
- Chung, E.; Rybalko, V.Y.; Hsieh, P.L.; Leal, S.L.; Samano, M.A.; Willauer, A.N.; Stowers, R.S.; Natesan, S.; Zamora, D.O.; Christy, R.J.; et al. Fibrin-based stem cell containing scaffold improves the dynamics of burn wound healing. Wound Repair Regen. 2016, 24, 810–819. [Google Scholar] [CrossRef]
- Lu, T.Y.; Yu, K.F.; Kuo, S.H.; Cheng, N.C.; Chuang, E.Y.; Yu, J.S. Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers 2020, 12, 2997. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef]
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.C.; Yang, K.C.; Lin, C.W.; Chen, Y.K.; Lu, T.Y.; Chen, H.Y.; Cheng, N.C.; Yu, J. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers 2019, 11, 1609. [Google Scholar] [CrossRef] [Green Version]
- Laiva, A.L.; O′Brien, F.J.; Keogh, M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Eng. Regen. Med. 2018, 12, 296–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascre, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Kim, Y.H.; Jun, Y.J.; Yoo, G.; Rhie, J.W. The effect of diabetes on the wound healing potential of adipose-tissue derived stem cells. Int. Wound J. 2016, 13 (Suppl. 1), 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, L.; Landau, S.; Flugelman, M.Y.; Levenberg, S. Genetically engineered human muscle transplant enhances murine host neovascularization and myogenesis. Commun. Biol. 2018, 1, 161. [Google Scholar] [CrossRef]
- Hsiao, C.T.; Cheng, H.W.; Huang, C.M.; Li, H.R.; Ou, M.H.; Huang, J.R.; Khoo, K.H.; Yu, H.W.; Chen, Y.Q.; Wang, Y.K.; et al. Fibronectin in cell adhesion and migration via N-glycosylation. Oncotarget 2017, 8, 70653–70668. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Assi, R.; Foster, R.T.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of msc in biomimetric engineered scaffolds promote healing of diabetic ulcers. Regen. Med. 2020, 11, 3–14. [Google Scholar]
- Li, X.; Chao, G.; Wang, L.; Xu, X.; Cai, Z.; Shi, L.; Zhuang, X.; Cheng, B. Preparation and BSA adsorption behavior of chitosan-arginine based nanofiber membranes. Fiber Polym. 2018, 19, 941–948. [Google Scholar] [CrossRef]
- Wang, X.; Rivera-Bolanos, N.; Jiang, B.; Ameer, G.A. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv. Funct. Mater. 2019, 29, 1809009. [Google Scholar] [CrossRef]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C.; Blau, H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nature 2018, 557, 335–342. [Google Scholar] [CrossRef]
- Lee, A.S.; Inayathullah, M.; Lijkwan, M.A.; Zhao, X.; Sun, W.; Park, S.; Hong, W.X.; Parekh, M.B.; Malkovskiy, A.V.; Lau, E.; et al. Prolonged survival of transplanted stem cells after ischaemic injury via the slow release of pro-survival peptides from a collagen matrix. Nat. Biomed. Eng. 2018, 2, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Xu, Z.; Wang, H.; Reese, B.E.; Gushchina, L.V.; Jiang, M.; Agarwal, P.; Xu, J.; Zhang, M.; Shen, R.; et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction. Nat. Commun. 2016, 7, 13306. [Google Scholar] [CrossRef]
- Barros, D.; Conde-Sousa, E.; Goncalves, A.M.; Han, W.M.; Garcia, A.J.; Amaral, I.F.; Pego, A.P. Engineering hydrogels with affinity-bound laminin as 3D neural stem cell culture systems. Biomateri. Sci. 2019, 7, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.; Young, S.; Gu, L.; Shah, N.; Lippens, E.; Weaver, J.; Duda, G.; Mooney, D. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv. Healthc. Mater. 2017, 6, 1601185. [Google Scholar] [CrossRef] [Green Version]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Nakamoto, T.; Dulinska-Molak, I.; Kawazoe, N.; Chen, G. Maintaining stemness of mesenchymal stem cells by tuning micro pattern features. J. Mater. Chem. B 2016, 4, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Kingshott, P.; Thissen, H.; Pera, M.; Wang, P.Y. Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: A review. Biotechnol. Bioeng. 2017, 114, 260–280. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin tissue substitutes and biomaterial risk assessment and testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]




| Fabrication Techniques | Advantages | Disadvantages | |
|---|---|---|---|
| Conventional fabrication techniques | Electrospinning | Essential for developing nanofibrous scaffolds, homogenous mixtures made of fibres with high tensile strength [24] | Process depends on many variables, problematic to obtain 3D structures with the required pore size needed for biomedical application [26,27] |
| Freeze drying | Used in a variety of purposes, capability of obtaining high temperature, manageable pore size by changing freezing method [24] | High energy consumption, long term timescale, generation of irregular size pores [28] | |
| Gas foaming | Porosity up to 56.71% [29] | Temperature dependent, product obtained from decreased temperature might have closed pore structure or a solid polymeric skin [30] | |
| Thermal induced phase separation | Porosity up to 80% [31], can use low temperature to integrate bioactive molecules [24] | Only used for polymers amenable to phase separation [31] | |
| Rapid prototyping (RP) | Bioprinting | Low cost, higher accuracy, and greater shape complexity [24] | Depends on the cells/biomaterials used [32] |
| Fused deposition modelling (FDM) | High tensile strength [24] | Has limited application to biodegradable polymers [33] | |
| Solvent based extrusion free forming (SEF) | Used to make ceramic, metal, and metal/ceramic composite part; used for precise control of scaffold structure at the micron level [24] | Variation in temperature affects extrusion pressure, including nozzle length-to-diameter ratio, and the extrusion velocity [34] | |
| Stereolithography | High resolution, uniformity in pore connectivity [24] | Requires a massive number of monomers and post-polymerization treatment to improve monomer conversion [35,36] | |
| Growth Factors | Origin of Secretion | Function | Study | Outcome |
|---|---|---|---|---|
| bFGF [39,45] | Endothelial cells, macrophages, monocytes | Stimulate proliferation, migration, and angiogenesis | In vivo | Improved re-epithelialization, angiogenesis, and collagen deposition in diabetic mice wound model |
| EGF [41,44] | Platelets, macrophages, fibroblasts | Epithelialization | In vivo | Enhanced granulated tissue formation, cell migration, and re-epithelialization in diabetic mice wound model |
| PDGF [42,45] | Platelets, keratinocytes, macrophages, endothelial cells, fibroblasts | Promotes cell proliferation, migration, and angiogenesis | In vivo | Enhanced granulation tissue formation and collagen deposition in full-thickness incision mice wound model |
| TGFβ3 [40,48] | Platelets, keratinocytes, macrophages, lymphocytes, fibroblasts | Inflammation, granulation tissue formation, epithelialization, matrix formation and remodeling | In vivo | Decreased scar formation in a rabbit model by reducing the ratio of type I to type III collagen |
| VGEF [43,45] | Platelets, macrophages, keratinocytes, endothelial cells | Epithelialization, collagen deposition, angiogenesis | In vivo | Promoted angiogenesis, collagen deposition, macrophage polarization and granulation tissue formation in full-thickness incision mice wound model |
| Types of Stem Cells | Wound Types | Mode of Delivery | Correction Time | Model Used | Treatment Effects |
|---|---|---|---|---|---|
| Adipose tissue derived MSCs [64] | Excisional wound | Scratch wound assay | 14 days | Rat | Accelerate migration and proliferation of fibroblast and keratinocytes |
| ASCs [67] | Full thickness wound | Transplantation | 14 days | Murine | Promote wound healing, reduce scar formation, and minimized long term side effects of cellular transplantation |
| ASCs [75] | Full thickness wound | Subdermal injection | 28 days | Rat | Increased tissue regeneration, suppression of inflammatory response, augmented EGF and VEGF production, promote re- epithelialization and cell infiltration |
| Autologous MSCs [65] | Cutaneous wound | Subjection | 14 days | Mouse | Promote wound repair by regaining wound tensile strength |
| BMSCs [71] | Excisional wound | Subcutaneous injection | 10 days | Mouse | Complete re-epithelialization and wound closure with a prominent keratinized layer |
| Human iPSCs [72] | Excisional wound | Intradermal injection | 14 days | Mouse | Promote angiogenesis, accelerated wound closure, and increased wound perfusion |
| Human iPSCs [21] | Excisional wound | Intravenous injection | 14 days | Mouse | Accelerated epithelialization |
| Types | Examples | Advantages | Disadvantages | Major Properties in Wound Healing |
|---|---|---|---|---|
| Natural biomaterials | Alginate [83,84] | Can retain its shape due to low viscosity and zero shear viscosity | Inert material and only suitable for in vitro assays, requires crosslinking due to low bioactivity | Porous, good absorption, biocompatible and biodegradable nature promote wound healing resulting in less scarring, minimal bacterial infection, and the creation of a moist wound environment |
| Cellulose [85,86] | Flexibility in shape, easy processing, good mechanical strength, and biodegradability | Lack of solubility in water and many organic solvents | Hydrophilic nature, purity, ability to maintain appropriate moisture balance and flexibility form a tight barrier between the wound and the environment, preventing bacterial infections | |
| Chitosan [78,87] | Possess antibacterial, antifungal, mucoadhesive and analgesic property | Poorly soluble in aqueous solutions except for acidic medium | Interact with negatively charged molecules (protein, fatty acid, bile acid, polysaccharide, phospholipids); chelate metal ions (iron, copper, magnesium); stimulate hemostasis and accelerate tissue regeneration | |
| Collagen [9,78,88] | Suitable mechanical property and biocompatibility | Susceptible to crosslinking and any sterilization procedure | Triple helix conformation of collagen type 1 favour cell adhesion and migration; pore sizes for the 5 and 8 mg/mL collagen type I scaffolds ranged between 126–188 μm promote connective tissue regeneration | |
| Elastin [78,89] | High elasticity | Poor mechanical strength and availability | Half-life > 70 years and the monomer can reversibly stretch up to eight times its resting length; fibre alignment positively affects cell phenotype, adhesion, and proliferation | |
| Fibrin [78,90] | Good protein binding ability that promotes vascularization | Limited control over its structural and mechanical properties | Fibrin network serves as a provisional template for promoting cell migration and proliferation; releases cytokines and growth factors attracting inflammatory cells at the wound bed; activates re-epithelialization, angiogenesis, connective tissue formation and contraction | |
| Gelatin [2,82,91] | Low antigenicity and higher solubility in solvents | Lack high mechanical resistance | Porous gelatin matrices absorb wound exudates, maintain a moist environment essential for wound healing | |
| Silk fibroin [83,92] | Biocompatible with strong mechanical properties | High brittleness | Porous template supports cell proliferation, differentiation, and ECM production | |
| Synthetic biomaterials | PCL [93,94] | Biocompatible with relatively slow degradation time | Poor cell attachment due to hydrophobicity | Show desirable electroactivity, biocompatibility, free radical scavenging capacity and antibacterial activity; promoted collagen deposition and granulation tissue thickness during the process of wound healing |
| PEG [9,78,95] | Reasonable control over structural and compositional properties | Lacks interactive cell character | Demonstrate biocompatible property, protein resistance, non-immunogenicity, non-toxicity, and good water solubility required for chronic wound healing | |
| PGA [9,78,96] | Highly biocompatible and biodegradable | Rapid mechanical strength loss | Exhibit reasonable wetting time, preferable surface morphology, low moisture uptake and prolonged swelling behavior | |
| PHA [97,98,99] | Low acidity and bioactivity, nontoxic degradation, biocompatibility, and non-carcinogenicity | Poor mechanical properties, high production cost, limited functionalities, incompatibility with conventional thermal processing techniques | Structural porosity and wettability similar to natural ECM, effectively promoting cellular migration, attachment, and proliferation | |
| PLA [96,100] | Easy modification with other biomaterials and bioactive compounds | Poor cell interaction, low elongation, and hydrophobicity | Exhibit high mechanical properties, reasonable wetting time, preferable surface morphology, low moisture uptake, prolonged swelling behavior and strong antibacterial properties against Staphylococcus aureus and Escherichia coli | |
| PLGA [9,78,101] | Biocompatible and biodegradable with a wide range of erosion time | Generates adverse inflammatory reaction upon degradation | Exhibit cytocompatibility and facilitate cell adhesion, spreading and proliferation, release anti-inflammatory factors required for wound healing accelerate collagen deposition and re-epithelialization |
| Biomaterials Used | Fabrication Method | Stem Cells | Application | Correction Time | Treatment Outcome |
|---|---|---|---|---|---|
| Chitosan & arginine based polyester amide [114] | Gel | MSC | 3rd degree burn wounds in a mouse model | 7 days | Promoted wound closure, re-epithelialization, granulation tissue growth, and blood vessel regeneration |
| Collagen [115] | Scaffold | hiPSC-SMC | Full-thickness cutaneous diabetic mouse wound | 7 days | Increased cellular proliferation, expression of pro-angiogenic and regenerative cytokines and angiogenesis |
| Collagen with stromal-derived factor-1 alpha (SDF-1α) gene [116] | Scaffold | ADSC | A non-healing diabetic foot ulcer | 14 days | Restore the pro-angiogenic regenerative response in the human diabetic ADSCs and exhibited active-matrix remodelling of fibronectin and basement membrane protein collagen IV |
| Fibrin [117] | Gel | ASC | Rat skins burn model | 7 days | Enhanced local angiogenesis of regenerating burn wound without impeding wound closure kinetics up to 21 days, integrates with wound surface allowing ASC transmigration into the regenerating wound and enhanced granulation tissue formation. |
| Gelatine [118] | Hydrogel | ASC | Murine burn model | 14 days | Highest wound contraction rate of 55.3%, decreased discoloration rating, roughness score and reduced scab formation |
| PCL [119] | Nanofibrous scaffold | BMSC | Full-thickness excisional wound in diabetic mouse | 7 days | Enhanced granulation tissue formation, angiogenesis, ECM deposition and elicited pro-regenerative response to accelerate wound healing |
| PEG [120] | Hydrogel | ADSC | Full-thickness excisional wound in the diabetic rat model | 7 days | Inhibit inflammation, promote angiogenesis and re-epithelialization |
| PLGA [121] | Nanofibrous scaffold | hASC | Full thickness excisional wound in mouse model | 7 days | Better cell activity in the PLGA matrix in terms of cell adhesion, proliferation, and survival along with improved wound healing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riha, S.M.; Maarof, M.; Fauzi, M.B. Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polymers 2021, 13, 1546. https://doi.org/10.3390/polym13101546
Riha SM, Maarof M, Fauzi MB. Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polymers. 2021; 13(10):1546. https://doi.org/10.3390/polym13101546
Chicago/Turabian StyleRiha, Shaima Maliha, Manira Maarof, and Mh Busra Fauzi. 2021. "Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review" Polymers 13, no. 10: 1546. https://doi.org/10.3390/polym13101546
APA StyleRiha, S. M., Maarof, M., & Fauzi, M. B. (2021). Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polymers, 13(10), 1546. https://doi.org/10.3390/polym13101546