In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Matrices Description
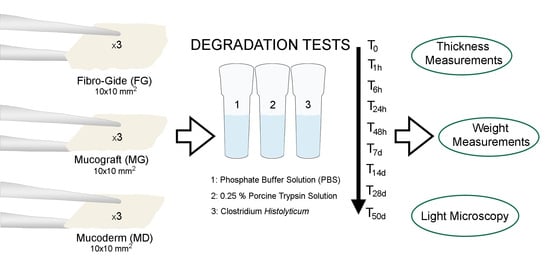
2.2. Degradation Assays
- (1)
- Hydrolytic degradation test: immersion of specimens in phosphate buffer solution (PBS) at 37 °C [37].
- (2)
- Enzyme resistance test: samples were immersed in a 0.25% porcine trypsin solution (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C [31].
- (3)
- Bacterial collagenase resistance test: collagenase solution from Clostridium histolyticum bacteria Type V (Sigma-Aldrich, St Louis, MO, USA) was used. This solution contained a mixture of several different enzymes including collagenase, which act together to break down tissue. This preparation contains collagenase, non-specific proteases, clostripain, neutral protease, and aminopeptidase activities. Specific activity is ≥125 CDU/mg solid. A collagenase concentration of 2 IU/mL in 50 mM Tris HCl (pH = 7.4), containing 10 mM CaCl2, was used [19,24]. After each 48 h, degradation solutions were carefully removed through suction and renewed [38].
2.3. Statistical Analysis
2.4. Light Microscopy Analysis
3. Results
3.1. Thickness Evaluation after PBS Degradation Assay
3.2. Thickness Evaluation after Trypsin Degradation Assay
3.3. Thickness Evaluation after C. histolyticum Collagenase Degradation Assay
3.4. Weight Evaluation after PBS Degradation Assay
3.5. Weight Evaluation after Trypsin Degradation Assay
3.6. Weight Evaluation after C. histolyticum Collagenase Degradation Assay
3.7. Matrices Morphological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolff, J.; Farré-Guasch, E.; Sándor, G.K.; Gibbs, S.; Jager, D.J.; Forouzanfar, T. Soft Tissue Augmentation Techniques and Materials Used in the Oral Cavity: An Overview. Implant Dent. 2016, 25, 427–434. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Jung, R.E.; Schwarz, F.; Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-Based Knowledge on the Aesthetics and Maintenance of Peri-Implant Soft Tissues: Osteology Foundation Consensus Report Part 1-Effects of Soft Tissue Augmentation Procedures on the Maintenance of Peri-Implant Soft Tissue Health. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 7–10. [Google Scholar] [CrossRef]
- Bassetti, R.G.; Stähli, A.; Bassetti, M.A.; Sculean, A. Soft Tissue Augmentation around Osseointegrated and Uncovered Dental Implants: A Systematic Review. Clin. Oral Investig. 2017, 21, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, N.P.; Löe, H. The Relationship between the Width of Keratinized Gingiva and Gingival Health. J. Periodontol. 1972, 43, 623–627. [Google Scholar] [CrossRef]
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical Evaluation of a New Collagen Matrix (Mucograft Prototype) to Enhance the Width of Keratinized Tissue in Patients with Fixed Prosthetic Restorations: A Randomized Prospective Clinical Trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Miyasato, M.; Crigger, M.; Egelberg, J. Gingival Condition in Areas of Minimal and Appreciable Width of Keratinized Gingiva. J. Clin. Periodontol. 1977, 4, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Matta, R.E.; Moest, T.; Humann, J.; Gammel, L.; Neukam, F.W.; Schlegel, K.A. Soft Tissue Volume Alterations after Connective Tissue Grafting at Teeth: The Subepithelial Autologous Connective Tissue Graft versus a Porcine Collagen Matrix–A Pre-Clinical Volumetric Analysis. J. Clin. Periodontol. 2016, 43, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Mounssif, I. Periodontal Plastic Surgery. Periodontology 2015, 68, 333–368. [Google Scholar] [CrossRef]
- Bassetti, M.; Kaufmann, R.; Salvi, G.E.; Sculean, A.; Bassetti, R. Soft Tissue Grafting to Improve the Attached Mucosa at Dental Implants: A Review of the Literature and Proposal of a Decision Tree. Quintessence Int. Berl. Ger. 1985 2015, 46, 499–510. [Google Scholar] [CrossRef]
- Definition and Objectives of Periodontal Plastic Surgery-Practical Periodontal Plastic Surgery-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119014775.ch1 (accessed on 27 December 2020).
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, Á.; Vallecillo, C.; Lynch, C.D.; Osorio, M.T.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part I: Natural Polymers-Based Biomaterials. Polymers 2020, 12, 1850. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Randomized Controlled Clinical Study Evaluating Effectiveness and Safety of a Volume-Stable Collagen Matrix Compared to Autogenous Connective Tissue Grafts for Soft Tissue Augmentation at Implant Sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Rothamel, D.; Benner, M.; Fienitz, T.; Happe, A.; Kreppel, M.; Nickenig, H.-J.; Zöller, J.E. Biodegradation Pattern and Tissue Integration of Native and Cross-Linked Porcine Collagen Soft Tissue Augmentation Matrices-an Experimental Study in the Rat. Head Face Med. 2014, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Pabst, A.M.; Lehmann, K.-M.; Walter, C.; Krüger, M.; Stratul, S.-I.; Kasaj, A. Influence of Porcine-Derived Collagen Matrix on Endothelial Progenitor Cells: An in Vitro Study. Odontology 2016, 104, 19–26. [Google Scholar] [CrossRef]
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a Porcine Collagen Matrix as an Alternative to Autogenous Tissue for Grafting Oral Soft Tissue Defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470. [Google Scholar] [CrossRef]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Rodriguez-Archilla, A.; Osorio, R. Collagen Matrix vs. Autogenous Connective Tissue Graft for Soft Tissue Augmentation: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 1810. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier Membranes: More than the Barrier Effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarman-Smith, M.L.; Bodamyali, T.; Stevens, C.; Howell, J.A.; Horrocks, M.; Chaudhuri, J.B. Porcine Collagen Crosslinking, Degradation and Its Capability for Fibroblast Adhesion and Proliferation. J. Mater. Sci. Mater. Med. 2004, 15, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Kohavi, D.; Krausz, E.; Steinberg, D.; Rosen, G. Enzymatic Degradation of Collagen-Guided Tissue Regeneration Membranes by Periodontal Bacteria. Clin. Oral Implant. Res. 2003, 14, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Calciolari, E.; Ravanetti, F.; Strange, A.; Mardas, N.; Bozec, L.; Cacchioli, A.; Kostomitsopoulos, N.; Donos, N. Degradation Pattern of a Porcine Collagen Membrane in an in Vivo Model of Guided Bone Regeneration. J. Periodontal. Res. 2018, 53, 430–439. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Aboodi, G.; Moses, O.; Tal, H.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Bio-Degradation of a Resorbable Collagen Membrane (Bio-Gide) Applied in a Double-Layer Technique in Rats. Clin. Oral Implant. Res. 2009, 20, 1116–1123. [Google Scholar] [CrossRef]
- von Arx, T.; Buser, D. Horizontal Ridge Augmentation Using Autogenous Block Grafts and the Guided Bone Regeneration Technique with Collagen Membranes: A Clinical Study with 42 Patients. Clin. Oral Implant. Res. 2006, 17, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in Degradation Behavior of Two Non-Cross-Linked Collagen Barrier Membranes: An in Vitro and in Vivo Study. Clin. Oral Implant. Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. Current Methods of Collagen Cross-Linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Lai, J.-Y. Photo-Cross-Linking of Amniotic Membranes for Limbal Epithelial Cell Cultivation. Mater. Sci. Eng. C 2014, 45, 313–319. [Google Scholar] [CrossRef]
- Lai, J.-Y. Interrelationship between Cross-Linking Structure, Molecular Stability, and Cytocompatibility of Amniotic Membranes Cross-Linked with Glutaraldehyde of Varying Concentrations. RSC Adv. 2014, 4, 18871–18880. [Google Scholar] [CrossRef]
- Ma, D.H.-K.; Lai, J.-Y.; Cheng, H.-Y.; Tsai, C.-C.; Yeh, L.-K. Carbodiimide Cross-Linked Amniotic Membranes for Cultivation of Limbal Epithelial Cells. Biomaterials 2010, 31, 6647–6658. [Google Scholar] [CrossRef]
- Maiorana, C.; Pivetti, L.; Signorino, F.; Grossi, G.B.; Herford, A.S.; Beretta, M. The Efficacy of a Porcine Collagen Matrix in Keratinized Tissue Augmentation: A 5-Year Follow-up Study. Int. J. Implant Dent. 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Mater. Basel Switz. 2019, 12, 3721. [Google Scholar] [CrossRef] [Green Version]
- An, Y.Z.; Kim, Y.K.; Lim, S.M.; Heo, Y.K.; Kwon, M.K.; Cha, J.K.; Lee, J.S.; Jung, U.W.; Choi, S.H. Physiochemical Properties and Resorption Progress of Porcine Skin-Derived Collagen Membranes: In Vitro and in Vivo Analysis. Dent. Mater. J. 2018, 37, 332–340. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular Matrix-Based Scaffolding Technologies for Periodontal and Peri-Implant Soft Tissue Regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Ferrantino, L.; Bosshardt, D.; Nevins, M.; Santoro, G.; Simion, M.; Kim, D. Tissue Integration of a Volume-Stable Collagen Matrix in an Experimental Soft Tissue Augmentation Model. Int. J. Periodontics Restor. Dent. 2016, 36, 807–815. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.J.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; Stupp, S.I.; Sader, R.A.; Kirkpatrick, C.J. Evaluation of the Tissue Reaction to a New Bilayered Collagen Matrix in Vivo and Its Translation to the Clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
- Kasaj, A.; Levin, L.; Stratul, S.-I.; Götz, H.; Schlee, M.; Rütters, C.B.; Konerding, M.A.; Ackermann, M.; Willershausen, B.; Pabst, A.M. The Influence of Various Rehydration Protocols on Biomechanical Properties of Different Acellular Tissue Matrices. Clin. Oral Investig. 2016, 20, 1303–1315. [Google Scholar] [CrossRef]
- Nocini, P.F.; Castellani, R.; Zanotti, G.; Gelpi, F.; Covani, U.; Marconcini, S.; de Santis, D. Extensive Keratinized Tissue Augmentation during Implant Rehabilitation after Le Fort I Osteotomy: Using a New Porcine Collagen Membrane (Mucoderm). J. Craniofac. Surg. 2014, 25, 799–803. [Google Scholar] [CrossRef]
- Sunandhakumari, V.J.; Vidhyadharan, A.K.; Alim, A.; Kumar, D.; Ravindran, J.; Krishna, A.; Prasad, M. Fabrication and In Vitro Characterization of Bioactive Glass/Nano Hydroxyapatite Reinforced Electrospun Poly(ε-Caprolactone) Composite Membranes for Guided Tissue Regeneration. Bioeng. Basel Switz. 2018, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.; Yoganarasimha, S.; Carrico, C.; Madurantakam, P. Incorporation of Fibrin Matrix into Electrospun Membranes for Periodontal Wound Healing. Bioengineering 2019, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, C.A.; Avery, N.C.; Rodin, V.V.; Bailey, A.J. The Increase in Denaturation Temperature Following Cross-Linking of Collagen Is Caused by Dehydration of the Fibres. J. Mol. Biol. 2005, 346, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.I.; Ackermann, M.; Konerding, M.A.; Willershausen, B.; Kasaj, A. In Vitro and in Vivo Characterization of Porcine Acellular Dermal Matrix for Gingival Augmentation Procedures. J. Periodontal. Res. 2014, 49, 371–381. [Google Scholar] [CrossRef]
- Eppley, B.L. Experimental Assessment of the Revascularization of Acellular Human Dermis for Soft-Tissue Augmentation. Plast. Reconstr. Surg. 2001, 107, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hoyama, E.; Schellini, S.A.; Marques, M.E.A.; Rossa, R.; Padovani, C.R. A Comparison of Human and Porcine Acellular Dermal Tissues in the Subcutaneous Space of a Rat Model. Orbit 2005, 24, 249–255. [Google Scholar] [CrossRef]
- Richter, G.T.; Smith, J.E.; Spencer, H.J.; Fan, C.Y.; Vural, E. Histological Comparison of Implanted Cadaveric and Porcine Dermal Matrix Grafts. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2007, 137, 239–242. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Herrera-Vizcaíno, C.; Orlowska, A.; Willershausen, I.; Sader, R.; Miron, R.J.; Choukroun, J.; Ghanaati, S. Biologization of Collagen-Based Biomaterials Using Liquid-Platelet-Rich Fibrin: New Insights into Clinically Applicable Tissue Engineering. Materials 2019, 12, 3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, S.; Ogawa, H.; Yamakita, E.; Watanabe, S.; Suzuki, T.; Nakashima, S. Adsorption of Water to Collagen as Studied Using Infrared (IR) Microspectroscopy Combined with Relative Humidity Control System and Quartz Crystal Microbalance. Appl. Spectrosc. 2017, 71, 1621–1632. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Li, Y.-T. Functional Assessment of Cross-Linked Porous Gelatin Hydrogels for Bioengineered Cell Sheet Carriers. Biomacromolecules 2010, 11, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.G.; Wess, T.J. The Effects of Hydration on the Collagen and Gelatine Phases within Parchment Artefacts. Herit Sci. 2013, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Owens, K.W.; Yukna, R.A. Collagen Membrane Resorption in Dogs: A Comparative Study. Implant Dent. 2001, 10, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of Differently Cross-Linked Collagen Membranes: An Experimental Study in the Rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Preidl, R.H.M.; Reichert, S.; Coronel, T.V.; Kesting, M.; Wehrhan, F.; Schmitt, C.M. Free Gingival Graft and Collagen Matrix Revascularization in an Enoral Open Wound Situation. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2021, 79, 1027–1037. [Google Scholar] [CrossRef]
- Arora, S.; Gordon, J.; Hook, M. Collagen Binding Proteins of Gram-Positive Pathogens. Front. Microbiol. 2021, 12, 628798. [Google Scholar] [CrossRef]
- Madani, A.; Garakani, K.; Mofrad, M.R.K. Molecular Mechanics of Staphylococcus Aureus Adhesin, CNA, and the Inhibition of Bacterial Adhesion by Stretching Collagen. PLoS ONE 2017, 12, e0179601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holderbaum, D.; Hall, G.S.; Ehrhart, L.A. Collagen Binding to Staphylococcus Aureus. Infect. Immun. 1986, 54, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Tay, F.C.M.; Ohno, H.; Sano, H.; Kaga, M.; Yiu, C.; Kumagai, H.; Kudou, Y.; Kubota, M.; Oguchi, H. SEM and TEM Analysis of Water Degradation of Human Dentinal Collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66, 287–298. [Google Scholar] [CrossRef] [PubMed]





| Matrix | Collagen | Origin | Cross-Link | Structure | Thickness (mm) | Exposed to the Oral Environment |
|---|---|---|---|---|---|---|
| Fibro-Gide | Type I and III | Porcine | Yes | 3D matrix | 6 | No Submerged healing |
| Mucograft | Type I and III | Porcine | No | 2 layers: compact and spongious scaffold | 2.5–5.0 | No/Yes Submerged healing recommended |
| Mucoderm | Type I and III | Porcine | No | 1 layer | 1.2–1.7 | Yes Open healing is feasible in the case of a vestibuloplasty. |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fibro-Gide | Mucograft | Mucoderm | |||||||
| Trypsin | C. histolyticum | PBS | Trypsin | C. histolyticum | PBS | Trypsin | C. histolyticum | PBS | |
| t0 | 5.78 (0.19) | 6.32 (0.12) | 5.68 (0.22) | 3.93 (1.58) | 3.55 (0.74) | 4.12 (0.43) | 1.39 (0.08) | 1.28 (0.18) | 1.22 (0.16) |
| 1 h | 3.84 (0.59) | 1.92 (0.35) | 3.91 (0.17) | 1.58 (1.99) | 0.18 (0.01) | 0.49 (0.12) | 1.15 (0.23) | 0.84 (0.12) | 0.88 (0.1) |
| 6 h | 2.48 (0.07) | 0.74 (0.18) | 3.34 (0.48) | 0.93 (1.19) | 0.13 (0.07) | 0.29 (0.059) | 0.81 (0.07) | 0.75 (0.05) | 0.67 (0.19) |
| 24 h | 2.6 (0.16) | 0.39 (0.19) | 1.77 (0.35) | 0.86 (1.15) | 0.12 (0.08) | 0.17 (0.07) | 0.65 (0.05) | 0.4 (0.02) | 0.62 (0.06) |
| 48 h | 2.51 (0.22) | 0.063 (0.095) | 1.52 (0.39) | 0.81 (1.08) | 0 (0) | 0.16 (0.02) | 0.42 (0.05) | 0 (0) | 0.49 (0.05) |
| 7 d | 1.48 (0.12) | 0.043 (0.065) | 1.18 (0.56) | 0.55 (0.69) | 0 (0) | 0.099 (0.027) | 0.17 (0.02) | 0 (0) | 0.16 (0.06) |
| 14 d | 0.37 (0.19) | 0 (0) | 0.47 (0.27) | 0.11 (0.09) | 0 (0) | 0.09 (0.008) | 0.02 (0.01) | 0 (0) | 0 (0) |
| 28 d | 0.21 (0.06) | 0 (0) | 0.36 (0.18) | 0.053 (0.08) | 0 (0) | 0 (0) | 0.01 (0.02) | 0 (0) | 0 (0) |
| 50 d | 0.12 (0.08) | 0 (0) | 0.35 (0.2) | 0.016 (0.02) | 0 (0) | 0 (0) | 0.01 (0.02) | 0 (0) | 0 (0) |
| (b) | |||||||||
| Th0–Th1h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 | 0.002 | 0.002 |
| Th0–Th6h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0–Th24h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0–Th48h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0–Th7d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0–Th14d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0–Th28d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Th0–Th50d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fibro-Gide | Mucograft | Mucoderm | |||||||
| Trypsin | C. histolyticum | PBS | Trypsin | C. histolyticum | PBS | Trypsin | C. histolyticum | PBS | |
| t0 | 43.5 (1.38) | 49.54 (1.97) | 45.55 (2.79) | 31.94 (9.63) | 26.97 (5.33) | 31.68 (5.02) | 56.34 (2.13) | 57.4 (3.8) | 41.63 (1.67) |
| 1 h | 44.11 (1.77) | 60.62 (2.93) | 53.31 (3.62) | 26.63 (14.24) | 12.64 (4.42) | 28.53 (5.28) | 55.09 (2.09) | 58.51 (3.77) | 42.1 (1.59) |
| 6 h | 39.15 (2.28) | 41.31 (2.48) | 48.99 (1.78) | 19.32 (16.18) | 3 (1.78) | 19.49 (3.08) | 54.09 (2.53) | 48.16 (3.75) | 39.95 (1.86) |
| 24 h | 27.78 (1.26) | 17.61 (0.86) | 37 (2.51) | 13.59 (11.84) | 0.57 (0.86) | 12.07 (7.67) | 43.51 (1.7) | 22.8 (2.75) | 37.23 (1.36) |
| 48 h | 20.35 (1.06) | 1.49 (2.24) | 24.03 (1.24) | 8.9 (8.5) | 0 (0) | 7.46 (4.77) | 30.64 (2.36) | 0 (0) | 29.14 (2.63) |
| 7 d | 11.61 (0.56) | 0.35 (0.53) | 18.64 (1.41) | 5.14 (5.38) | 0 (0) | 3.91 (2.45) | 10.07 (1.19) | 0 (0) | 6.48 (5.77) |
| 14 d | 3.8 (0.39) | 0 (0) | 14.39 (2.41) | 1.47 (1.75) | 0 (0) | 1.97 (1.09) | 0.21 (0.16) | 0 (0) | 0 (0) |
| 28 d | 1.85 (0.32) | 0 (0) | 10.31 (1.71) | 0.52 (0.77) | 0 (0) | 0 (0) | 0.08 (0.13) | 0 (0) | 0 (0) |
| 50 d | 0.99 (0.3) | 0 (0) | 7.62 (1.06) | 0.26 (0.39) | 0 (0) | 0 (0) | 0.16 (0.25) | 0 (0) | 0 (0) |
| (b) | |||||||||
| W0–W1h | 0.002 | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W6h | <0.001 | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W24h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W48h | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W7d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W14d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W28d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| W0–W50d | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation. Polymers 2021, 13, 2633. https://doi.org/10.3390/polym13162633
Vallecillo C, Toledano-Osorio M, Vallecillo-Rivas M, Toledano M, Osorio R. In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation. Polymers. 2021; 13(16):2633. https://doi.org/10.3390/polym13162633
Chicago/Turabian StyleVallecillo, Cristina, Manuel Toledano-Osorio, Marta Vallecillo-Rivas, Manuel Toledano, and Raquel Osorio. 2021. "In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation" Polymers 13, no. 16: 2633. https://doi.org/10.3390/polym13162633
APA StyleVallecillo, C., Toledano-Osorio, M., Vallecillo-Rivas, M., Toledano, M., & Osorio, R. (2021). In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation. Polymers, 13(16), 2633. https://doi.org/10.3390/polym13162633