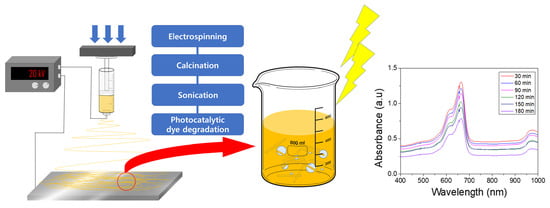
Fabrication and Photocatalytic Properties of Electrospun Fe-Doped TiO2 Nanofibers Using Polyvinyl Pyrrolidone Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Electrospinning Process
2.3. Characterization
2.4. Photocatalytic Degradation Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-T.; Choi, W.-Y. Fabrication of TiO2 photonic crystal by anodic oxidation and their optical sensing properties. Sensors Actuators A Phys. 2017, 260, 178–184. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Swart, H.C.; Motaung, D.E. Fabrication of TiO2 nanofibers based sensors for enhanced CH4 performance induced by notable surface area and acid treatment. Vacuum 2021, 187, 110102. [Google Scholar] [CrossRef]
- Li, F.; Song, H.; Yu, W.; Ma, Q.; Dong, X.; Wang, J.; Liu, G. Electrospun TiO2//SnO2 Janus nanofibers and its application in ethanol sensing. Mater. Lett. 2020, 262, 127070. [Google Scholar] [CrossRef]
- Kim, W.-T.; Na, K.-H.; Lee, J.-K.; Jang, I.; Choi, D.-S.; Choi, W.-Y. Porous TiO2 Nanotube Arrays for Drug Loading and Their Elution Sensing. J. Nanosci. Nanotechnol. 2019, 19, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, C.; Xiao, X. Polydopamine modified TiO2 nanotube arrays as a local drug delivery system for ibuprofen. J. Drug Deliv. Sci. Technol. 2020, 56, 101537. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Liu, D.; Bi, Y. Controllable fabrication of hollow TiO2 spheres as sustained release drug carrier. Adv. Powder Technol. 2019, 30, 2169–2177. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Yi, X.; Yu, H. Edge-selectively amidated graphene for boosting H2-evolution activity of TiO2 photocatalyst. Appl. Catal. B Environ. 2020, 264, 118504. [Google Scholar] [CrossRef]
- Khan, T.T.; Bari, G.A.K.M.; Kang, H.-J.; Lee, T.-G.; Park, J.-W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.-J.; Ou, N.; Lyu, B.; Gui, B.; Tian, S.; Qian, D.; Wang, X.; Yang, J. Visible-light driven TiO2 photocatalyst coated with graphene quantum dots of tunable nitrogen doping. Molecules 2019, 24, 344. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Wang, J.; Zhou, J.; Xi, Q.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Fabricating I doped TiO2 photoelectrode for the degradation of diclofenac: Performance and mechanism study. Chem. Eng. J. 2019, 369, 968–978. [Google Scholar] [CrossRef]
- Kim, K.-P.; Kim, W.H.; Kwon, S.M.; Kim, J.Y.; Do, Y.S.; Woo, S. Enhanced Light Absorption by Facile Patterning of Nano-Grating on Mesoporous TiO2 Photoelectrode for Cesium Lead Halide Perovskite Solar Cells. Nanomaterials 2021, 11, 1233. [Google Scholar] [CrossRef]
- Tsvetkov, N.; Larina, L.; Ku Kang, J.; Shevaleevskiy, O. Sol-gel processed TiO2 nanotube photoelectrodes for dye-sensitized solar cells with enhanced photovoltaic performance. Nanomaterials 2020, 10, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, M.M.; Rasheed, M. Solid State Reaction Synthesis and Characterization of Cu doped TiO2 Nanomaterials. In Proceedings of the 2nd International Conference on Materials, Laser science and Applied physics (ICMLAP), Orlando, FL, USA, 10–14 October; 2021; 1795, p. 12059. [Google Scholar]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er Co-doped TiO2 for rapid bacteria-killing using visible light. Bioact. Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Xu, S.; Zhou, Y.; Wang, X.; Peng, H.; Du, J. Preparation of Gd-Doped TiO2 Nanotube Arrays by Anodization Method and Its Photocatalytic Activity for Methyl Orange Degradation. Catalysts 2020, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.A.; Hedhili, M.N.; Anjum, D.H.; Singaravelu, V.; Chung, S.H. Synthesis and Characterization of Iron-Doped TiO2 Nanoparticles Using Ferrocene from Flame Spray Pyrolysis. Catalysts 2021, 11, 438. [Google Scholar] [CrossRef]
- Heng, J.Z.X.; Tang, K.Y.; Regulacio, M.D.; Lin, M.; Loh, X.J.; Li, Z.; Ye, E. Solar-powered photodegradation of pollutant dyes using silver-embedded porous TiO2 nanofibers. Nanomaterials 2021, 11, 856. [Google Scholar] [CrossRef]
- Tang, K.Y.; Chen, J.X.; Legaspi, E.D.R.; Owh, C.; Lin, M.; Tee, I.S.Y.; Kai, D.; Loh, X.J.; Li, Z.; Regulacio, M.D.; et al. Gold-decorated TiO2 nanofibrous hybrid for improved solar-driven photocatalytic pollutant degradation. Chemosphere 2021, 265, 129114. [Google Scholar] [CrossRef] [PubMed]
- Valero-Romero, M.J.; Santaclara, J.G.; Oar-Arteta, L.; van Koppen, L.; Osadchii, D.Y.; Gascon, J.; Kapteijn, F. Photocatalytic properties of TiO2 and Fe-doped TiO2 prepared by metal organic framework-mediated synthesis. Chem. Eng. J. 2019, 360, 75–88. [Google Scholar] [CrossRef]
- Ruggieri, F.; Di Camillo, D.; Maccarone, L.; Santucci, S.; Lozzi, L. Electrospun Cu-, W- and Fe-doped TiO2 nanofibres for photocatalytic degradation of rhodamine 6G. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.-L.; Hao, H.; Lang, X. Visible light-induced selective oxidation of alcohols with air by dye-sensitized TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 232, 260–267. [Google Scholar] [CrossRef]
- Ding, H.; Xu, M.; Zhang, S.; Yu, F.; Kong, K.; Shen, Z.; Hua, J. Organic blue-colored DA-π-A dye-sensitized TiO2 for efficient and stable photocatalytic hydrogen evolution under visible/near-infrared-light irradiation. Renew. Energy 2020, 155, 1051–1059. [Google Scholar] [CrossRef]
- Ahmad, I.; Kan, C. Visible-light-driven, dye-sensitized TiO2 photo-catalyst for self-cleaning cotton fabrics. Coatings 2017, 7, 192. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-Q.; Mo, L.-E.; Chen, W.-C.; Shi, X.-Q.; Wang, N.; Hu, L.-H.; Hayat, T.; Alsaedi, A.; Dai, S.-Y. Solvothermal synthesis of hierarchical TiO2 microstructures with high crystallinity and superior light scattering for high-performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2017, 9, 32026–32033. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Wang, L.; Liu, R.; Zeng, Y.; Xia, X.; Liu, Y.; Luo, S. Facile synthesis of bird’s nest-like TiO2 microstructure with exposed (001) facets for photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 391, 228–235. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Li, T. A study of controllable synthesis and formation mechanism on flower-like TiO2 with spherical structure. Crystals 2018, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Akshay, V.R.; Arun, B.; Mandal, G.; Mutta, G.R.; Chanda, A.; Vasundhara, M. Observation of optical band-gap narrowing and enhanced magnetic moment in co-doped sol–gel-derived anatase TiO2 nanocrystals. J. Phys. Chem. C 2018, 122, 26592–26604. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Sharma, S.; Singh, R.C. Effect of tungsten doping on structural and optical properties of rutile TiO2 and band gap narrowing. Optik 2019, 182, 538–547. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Lin, Y.-H.; Wang, P.W. X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Doped TiO2 Films Prepared by Reactive-Ion-Beam Sputtering with Various NH3/O2 Gas Mixture Ratios. Coatings 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef] [Green Version]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Kumaravel, V.; Rhatigan, S.; Mathew, S.; Michel, M.C.; Bartlett, J.; Nolan, M.; Hinder, S.J.; Gascó, A.; Ruiz-Palomar, C.; Hermosilla, D. Mo doped TiO2: Impact on oxygen vacancies, anatase phase stability and photocatalytic activity. J. Phys. Mater. 2020, 3, 25008. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Begum, T.; Gogoi, P.K.; Bora, U. Photocatalytic degradation of crystal violet dye on the surface of Au doped TiO2 nanoparticle. Indian J. Chem. Technol. 2017, 24, 97–101. [Google Scholar]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly efficient low-temperature N-doped TiO2 catalysts for visible light photocatalytic applications. Materials. 2018, 11, 584. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Simsek, E.B. Solvothermal synthesized boron doped TiO2 catalysts: Photocatalytic degradation of endocrine disrupting compounds and pharmaceuticals under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 309–322. [Google Scholar] [CrossRef]
- Qin, D.-D.; Wang, Q.-H.; Chen, J.; He, C.-H.; Li, Y.; Wang, C.-H.; Quan, J.-J.; Tao, C.-L.; Lu, X.-Q. Phosphorus-doped TiO2 nanotube arrays for visible-light-driven photoelectrochemical water oxidation. Sustain. Energy Fuels 2017, 1, 248–253. [Google Scholar] [CrossRef]
- Yang, C.; Shang, S.; Li, X. Fabrication of sulfur-doped TiO2 nanotube array as a conductive interlayer of PbO2 anode for efficient electrochemical oxidation of organic pollutants. Sep. Purif. Technol. 2021, 258, 118035. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Chen, S.-S.; Yang, S.-Y.; Liu, H.-M.; Lin, K.-S. Sol-Gel hydrothermal synthesis and visible light photocatalytic degradation performance of Fe/N codoped TiO2 catalysts. Materials. 2018, 11, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The preparation of TiO2 film by the sol-gel method and evaluation of its self-cleaning property. Materials 2018, 11, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çırak, B.B.; Karadeniz, S.M.; Kılınç, T.; Caglar, B.; Ekinci, A.E.; Yelgin, H.; Kürekçi, M.; Çırak, Ç. Synthesis, surface properties, crystal structure and dye sensitized solar cell performance of TiO2 nanotube arrays anodized under different voltages. Vacuum 2017, 144, 183–189. [Google Scholar] [CrossRef]
- Kim, W.-T.; Na, K.-H.; Park, D.-C.; Yang, W.-H.; Choi, W.-Y. Photocatalytic Methylene Blue Degradation of Electrospun Ti–Zn Complex Oxide Nanofibers. Nanomaterials 2020, 10, 1311. [Google Scholar] [CrossRef]
- Lee, C.-G.; Na, K.-H.; Kim, W.-T.; Park, D.-C.; Yang, W.-H.; Choi, W.-Y. TiO2/ZnO Nanofibers Prepared by Electrospinning and Their Photocatalytic Degradation of Methylene Blue Compared with TiO2 Nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tian, L.; Zhu, T.; Mei, J.; Chen, Z.; Yu, D.-G. Electrospun aspirin/Eudragit/lipid hybrid nanofibers for colon-targeted delivery using an energy-saving process. Chem. Res. Chinese Univ. 2021, 1–7. [Google Scholar]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–shell eudragit s100 nanofibers prepared via triaxial electrospinning to provide a colon-targeted extended drug release. Polymers. 2020, 12, 2034. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus zein–PVP nanofibers provide a two-stage controlled release of poorly water-soluble drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Aidana, Y.; Wang, Y.; Li, J.; Chang, S.; Wang, K.; Yu, D.-G. Fast Dissolution Electrospun Medicated Nanofibers for Effective Delivery of Poorly Water-Soluble Drugs. Curr. Drug Deliv. 2021. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Green electrospining of hydroxypropyl cellulose nanofibres for drug delivery applications. J. Nanosci. Nanotechnol. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Eco-friendly technology for preparation, characterization and promotion of honey bee propolis extract loaded cellulose acetate nanofibers in medical domains. Cellulose 2018, 25, 5195–5204. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; El-Naggar, M.E.; Hudson, S.M.; Rojas, O.J. Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. Int. J. Biol. Macromol. 2017, 94, 96–105. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Abdelgawad, A.M.; Salas, C.; Rojas, O.J. Curdlan in fibers as carriers of tetracycline hydrochloride: Controlled release and antibacterial activity. Carbohydr. Polym. 2016, 154, 194–203. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Preparation of biocompatible system based on electrospun CMC/PVA nanofibers as controlled release carrier of diclofenac sodium. J. Macromol. Sci. Part A 2016, 53, 566–573. [Google Scholar] [CrossRef]
- Rubin Pedrazzo, A.; Cecone, C.; Morandi, S.; Manzoli, M.; Bracco, P.; Zanetti, M. Nanosized SnO2 Prepared by Electrospinning: Influence of the Polymer on Both Morphology and Microstructure. Polymers 2021, 13, 977. [Google Scholar] [CrossRef]
- Ehrmann, A. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Mirata, S.; Sionkowska, A.; Vicini, S.; Alloisio, M.; Castellano, M. Effect of crosslinking type on the physical-chemical properties and biocompatibility of chitosan-based electrospun membranes. Polymers 2021, 13, 831. [Google Scholar] [CrossRef]
- Chiaradia, V.; Hanay, S.B.; Kimmins, S.D.; de Oliveira, D.; Araújo, P.H.H.; Sayer, C.; Heise, A. Crosslinking of electrospun fibres from unsaturated polyesters by bis-triazolinediones (TAD). Polymers 2019, 11, 1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-Y.; Lee, J.-H.; Choi, D.-Y.; Hwang, C.-H.; Lee, J.-W. Influence of Fe doping on phase transformation and crystallite growth of electrospun TiO2 nanofibers for photocatalytic reaction. Mater. Lett. 2012, 88, 156–159. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile - Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günnemann, C.; Haisch, C.; Fleisch, M.; Schneider, J.; Emeline, A.V.; Bahnemann, D.W. Insights into Different Photocatalytic Oxidation Activities of Anatase, Brookite, and Rutile Single-Crystal Facets. ACS Catal. 2019, 9, 1001–1012. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R. V Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, K.-H.; Kim, B.-S.; Yoon, H.-S.; Song, T.-H.; Kim, S.-W.; Cho, C.-H.; Choi, W.-Y. Fabrication and Photocatalytic Properties of Electrospun Fe-Doped TiO2 Nanofibers Using Polyvinyl Pyrrolidone Precursors. Polymers 2021, 13, 2634. https://doi.org/10.3390/polym13162634
Na K-H, Kim B-S, Yoon H-S, Song T-H, Kim S-W, Cho C-H, Choi W-Y. Fabrication and Photocatalytic Properties of Electrospun Fe-Doped TiO2 Nanofibers Using Polyvinyl Pyrrolidone Precursors. Polymers. 2021; 13(16):2634. https://doi.org/10.3390/polym13162634
Chicago/Turabian StyleNa, Kyeong-Han, Bo-Sung Kim, Han-Sol Yoon, Tae-Hyeob Song, Sung-Wook Kim, Churl-Hee Cho, and Won-Youl Choi. 2021. "Fabrication and Photocatalytic Properties of Electrospun Fe-Doped TiO2 Nanofibers Using Polyvinyl Pyrrolidone Precursors" Polymers 13, no. 16: 2634. https://doi.org/10.3390/polym13162634
APA StyleNa, K. -H., Kim, B. -S., Yoon, H. -S., Song, T. -H., Kim, S. -W., Cho, C. -H., & Choi, W. -Y. (2021). Fabrication and Photocatalytic Properties of Electrospun Fe-Doped TiO2 Nanofibers Using Polyvinyl Pyrrolidone Precursors. Polymers, 13(16), 2634. https://doi.org/10.3390/polym13162634