Palladium-Catalyzed Mizoroki–Heck and Copper-Free Sonogashira Coupling Reactions in Water Using Thermoresponsive Polymer Micelles
Abstract
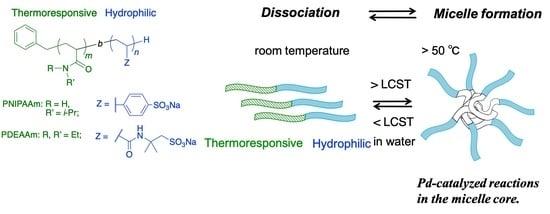
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Preparation of the Homopolymer PNIPAAm
2.3. Preparation of the Copolymer PNIPAAm-b-PAMPSNa NA-T
2.4. Removal of the Ethyl Xanthogenate Terminus in the PNIPAAm-b-PAMPSNa NA-T: Preparation of NA
2.5. Preparation of the Homopolymer PDEAAm
2.6. Preparation of the Copolymer PDEAAm-b-PSSNa DS-T
2.7. Removal of Trithiocarbonate Terminus from DS-T; Synthesis of DS
2.8. Preparation of the Diblock Copolymer Poly(DEAAm-b-AMPSNa) DA-T
2.9. Removal of Trithiocarbonate Terminus from DA-T; Synthesis of DA
2.10. Mizoroki–Heck Reactions in Water Using the Copolymers, Initial Study
2.11. Mizoroki–Heck Reactions in Water Using the Copolymers Catalyzed by 1
2.12. Evaluation of Extraction Efficiencies of Mizoroki–Heck Product 5aa
2.13. Sonogashira Coupling Reactions in Water Using the Copolymers
3. Results and Discussion
3.1. Preparation and Temperature-Dependent Properties of the Diblock Copolymers
3.2. Palladium-Catalyzed Mizoroki–Heck Reaction Using the Copolymers
3.3. Reuse of the Aqueous Solution and Formation of Palladium Nanoparticles (PdNPs)
3.4. Extraction Efficiency
3.5. Sonogashira Coupling Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A. The E Factor: Fifteen Years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Cortes-Clerget, M.; Yu, J.; Kincaid, J.R.A.; Walde, P.; Gallou, F.; Lipshutz, B.H. Water As the Reaction Medium in Organic Chemistry: From Our Worst Enemy to Our Best Friend. Chem. Sci. 2021, 12, 4237–4266. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Cortes-Clerget, M. The Hydrophobic Effect Applied to Organic Synthesis: Recent Synthetic Chemistry “In Water”. Chem. A Eur. J. 2018, 24, 6672–6695. [Google Scholar] [CrossRef] [PubMed]
- Christoffel, F.; Ward, T.R. Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review. Catal. Lett. 2017, 148, 489–511. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Gallou, F.; Handa, S. Evolution of Solvents in Organic Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S. Transitioning Organic Synthesis from Organic Solvents to Water. What’s E Factor? Green Chem. 2014, 16, 3660–3679. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Abela, A.R.; Bošković, Ž.V.; Nishikata, T.; Duplais, C.; Krasovskiy, A. “Greening Up” Cross-Coupling Chemistry. Top. Catal. 2010, 53, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Kitanosono, T.; Kobayashi, S. Reactions in Water Involving the “On-Water” Mechanism. Chem. A Eur. J. 2020, 26, 9408–9429. [Google Scholar] [CrossRef]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef]
- Guo, W.; Liu, X.; Liu, Y.; Li, C. Chiral Catalysis at the Water/Oil Interface. ACS Catal. 2017, 8, 328–341. [Google Scholar] [CrossRef]
- Uozumi, Y. Heterogeneous Asymmetric Catalysis in Water with Amphiphilic Polymer-Supported Homochiral Palladium Complexes. Bull. Chem. Soc. Jpn. 2008, 81, 1183–1195. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Hu, Y.; Yu, J.; Gallou, F.; Lipshutz, B.H. Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki-Heck Couplings under Aqueous Micellar Catalysis Conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.-C.; Fouquet, E.; Felpin, F.-X. Recyclable Heterogeneous Palladium Catalysts in Pure Water: Sustainable Developments in Suzuki, Heck, Sonogashira and Tsuji-Trost Reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Cordes, E.H.; Dunlap, R.B. Kinetics of Organic Reactions in Micellar Systems. Acc. Chem. Res. 2002, 2, 329–337. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Effects of Surfactants on the Rate of Chemical Reactions. J. Chem. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic Acceleration of Diels-Alder Reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Breslow, R. Hydrophobic Effects on Simple Organic Reactions in Water. Acc. Chem. Res. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Okano, T. Design of Temperature-Responsive Polymer-Grafted Surfaces for Cell Sheet Preparation and Manipulation. Bull. Chem. Soc. Jpn. 2019, 92, 817–824. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Ma, Y.; Gao, X.; Wang, X.; Dai, X.; Song, J. Application of Poly(N-isopropylacrylamide) as Thermosensitive Smart Materials. J. Phys. Conf. Ser. 2020, 1676, 012063. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Seki, T.; Takeoka, Y. Structural Colored Gels for Tunable Soft Photonic Crystals. Chem. Rec. 2009, 9, 87–105. [Google Scholar] [CrossRef]
- Hellweg, T. Responsive Core-Shell Microgels: Synthesis, Characterization, and Possible Applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1073–1083. [Google Scholar] [CrossRef]
- Hertle, Y.; Hellweg, T. Thermoresponsive Copolymer Microgels. J. Mater. Chem. B 2013, 1, 5874–5885. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L. Thermoresponsive Hydrogels in Biomedical Applications: A Seven-Year Update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H. Thermo-Responsive Polymer Gels. In Macromolecular Science and Engineering; Tanabe, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 71–83. [Google Scholar]
- Luo, G.-F.; Chen, W.-H.; Zhang, X.-Z. 100th Anniversary of Macromolecular Science Viewpoint: Poly(N-isopropylacrylamide)-Based Thermally Responsive Micelles. ACS Macro Lett. 2020, 9, 872–881. [Google Scholar] [CrossRef]
- Umekar, M.J.; Rarokar, N.R.; Tatode, A.A.; Agrawal, R.D. Polymeric Micelle as a Nanocarrier for Delivery of Therapeutic Agents: A Comprehensive Review. J. Drug Deliv. Ther. 2020, 10, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Okano, T. Intelligent Thermoresponsive Polymeric Micelles for Targeted Drug Delivery. J. Drug Deliv. Sci. Technol. 2006, 16, 35–44. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Suzuki, K.; Aoyagi, T.; Sakurai, Y.; Okano, T. Reversibly Thermo-Responsive Alkyl-Terminated Poly(N-Isopropylacrylamide) Core-Shell Micellar Structures. Colloids Surf. B Biointerfaces 1997, 9, 37–48. [Google Scholar] [CrossRef]
- Winnik, F.M.; Adronov, A.; Kitano, H. Pyrene-Labeled Amphiphilic Poly-(N-Isopropylacrylamides) Prepared by Using a Lipophilic Radical Initiator: Synthesis, Solution Properties in Water, and Interactions with Liposomes. Can. J. Chem. 1995, 73, 2030–2040. [Google Scholar] [CrossRef]
- Seto, H.; Matsumoto, H.; Miura, Y. Preparation of Palladium-Loaded Polymer Hydrogel Catalysts with High Durability and Recyclability. Polym. J. 2020, 52, 671–679. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. An Assembled Complex of Palladium and Non-Cross-linked Amphiphilic Polymer: A Highly Active and Recyclable Catalyst for the Suzuki-Miyaura Reaction. Org. Lett. 2002, 4, 3371–3374. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Suzuki, Y.; Yamada, Y.M.; Tabata, H.; Takahashi, H.; Ikegami, S. A Recyclable Catalytic System Based on a Temperature-Responsive Catalyst. Angew. Chem. Intl. Ed. Engl. 2005, 44, 4536–4538. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Suzuki, Y.; Takahashi, H.; Ikegami, S. Direct Transformation of Benzilic Amines to Carbonyls Using Polyacrylamide-Bound Tungstate under Phase-Transfer Catalysis Conditions. Tetrahedron Lett. 2007, 48, 4239–4242. [Google Scholar] [CrossRef]
- Ikegami, S.; Hamamoto, H. Novel Recycling System for Organic Synthesis Via Designer Polymer-Gel Catalysts. Chem. Rev. 2009, 109, 583–593. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, W.; Zhang, M. Pd-Catalyzed C-C Cross-Coupling Reactions within a Thermoresponsive and Ph-Responsive and Chelating Polymeric Hydrogel. J. Org. Chem. 2009, 74, 1923–1931. [Google Scholar] [CrossRef]
- Sato, T.; Ohno, A.; Sarkar, S.M.; Uozumi, Y.; Yamada, Y.M.A. A Convoluted Polymeric Imidazole Palladium Catalyst: Structural Elucidation and Investigation of the Driving Force for The Efficient Mizoroki-Heck Reaction. ChemCatChem 2015, 7, 2141–2148. [Google Scholar] [CrossRef]
- Yamada, Y.M.; Sarkar, S.M.; Uozumi, Y. Amphiphilic Self-Assembled Polymeric Copper Catalyst to Parts Per Million Levels: Click Chemistry. J. Am. Chem. Soc. 2012, 134, 9285–9290. [Google Scholar] [CrossRef]
- Yamada, Y.M.; Sarkar, S.M.; Uozumi, Y. Self-Assembled Poly(Imidazole-Palladium): Highly Active, Reusable Catalyst at Parts Per Million to Parts Per Billion Levels. J. Am. Chem. Soc. 2012, 134, 3190–3198. [Google Scholar] [CrossRef]
- Sarkar, S.M.; Uozumi, Y.; Yamada, Y.M. A Highly Active and Reusable Self-Assembled Poly(Imidazole/Palladium) Catalyst: Allylic Arylation/Alkenylation. Angew. Chem. Int. Ed. Engl. 2011, 50, 9437–9441. [Google Scholar] [CrossRef]
- Chen, T.; Fang, Q.; Zhou, L.; Xu, Z.; Qiu, J.; Wang, M.; Wang, J. Comparative Study of Cross-Linked and Linear Thermo-Responsive Carriers Supported Palladium Nanoparticles in the Reduction of 4-Nitrophenol: Structure, Catalytic Activity and Responsive Catalysis Property. React. Funct. Polym. 2019, 142, 104–111. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. Assembled Catalyst of Palladium and Non-Cross-Linked Amphiphilic Polymer Ligand for the Efficient Heterogeneous Heck Reaction. Tetrahedron 2004, 60, 4097–4105. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. An Efficient Heterogeneous Heck Reaction Promoted by a New Assembled Catalyst of Palladium and Non-Cross-Linked Amphiphilic Polymer. Tetrahedron Lett. 2003, 44, 2379–2382. [Google Scholar] [CrossRef]
- Suzuki, N.; Mizuno, D.; Guidote, A.M.; Koyama, S.; Masuyama, Y.; Rikukawa, M. Asymmetric Reactions in Water Catalyzed by L-Proline Tethered on Thermoresponsive Ionic Copolymers. Lett. Org. Chem. 2020, 17, 717–725. [Google Scholar] [CrossRef]
- Suzuki, N.; Takabe, T.; Yamauchi, Y.; Koyama, S.; Koike, R.; Rikukawa, M.; Liao, W.-T.; Peng, W.-S.; Tsai, F.-Y. Palladium-Catalyzed Mizoroki-Heck Reactions in Water Using Thermoresponsive Polymer Micelles. Tetrahedron 2019, 75, 1351–1358. [Google Scholar] [CrossRef]
- Suzuki, N.; Akebi, R.; Inoue, T.; Rikukawa, M.; Masuyama, Y. Asymmetric Aldol and Michael Reactions in Water Using Organocatalysts Immobilized on a Thermoresponsive “Linear” Block Copolymer. Curr. Organocatal. 2016, 3, 306–314. [Google Scholar] [CrossRef]
- Suzuki, N.; Inoue, T.; Asada, T.; Akebi, R.; Kobayashi, G.; Rikukawa, M.; Masuyama, Y.; Ogasawara, M.; Takahashi, T.; Thang, S.H. Asymmetric Aldol Reaction on Water Using an Organocatalyst Tethered on a Thermoresponsive Block Copolymer. Chem. Lett. 2013, 42, 1493–1495. [Google Scholar] [CrossRef]
- Zayas, H.A.; Lu, A.; Valade, D.; Amir, F.; Jia, Z.; O’Reilly, R.K.; Monteiro, M.J. Thermoresponsive Polymer-Supportedl-Proline Micelle Catalysts for the Direct Asymmetric Aldol Reaction in Water. ACS Macro Lett. 2013, 2, 327–331. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Li, M.-H.; Feng, L.; Zhou, H.-Y.; Wang, J.-X. Construction Of Reversible Nano Reactor by Thermo-Responsive Polymeric Surfactant: Its Application in Chloromethylation of Naphthalene. J. Environ. Chem. Eng. 2019, 7, 103034. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, Y.; Jia, D.-S.; Cui, Z.-M.; Song, W.-G. Simple Synthesis of Sub-Nanometer Pd Clusters: High Catalytic Activity of Pd/PEG-PNIPAM in Suzuki Reaction. Chin. J. Catal. 2017, 38, 651–657. [Google Scholar] [CrossRef]
- Dolya, N.A.; Kudaibergenov, S.E. Catalysis by Thermiresponsive Polymers. In Temperature-Responsive Polymers: Chemistry, Properties, and Applications; Khutoryanskiy, V.V., Georgiou, T.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 357–377. [Google Scholar]
- Hamasaka, G.; Ichii, S.; Uozumi, Y. A Palladium NNC-Pincer Complex as an Efficient Catalyst Precursor for the Mizoroki-Heck Reaction. Adv. Synth. Catal. 2018, 360, 1833–1840. [Google Scholar] [CrossRef]
- Zehm, D.; Laschewsky, A.; Gradzielski, M.; Prevost, S.; Liang, H.; Rabe, J.P.; Schweins, R.; Gummel, J. Amphiphilic Dual Brush Block Copolymers as “Giant Surfactants” and Their Aqueous Self-Assembly. Langmuir 2010, 26, 3145–3155. [Google Scholar] [CrossRef]
- Flynn, S.; Dale, S.D.; Dwyer, A.B.; Chambon, P.; Rannard, S.P. In Situ Xanthate Deprotection to Generate Thiol Chain Transfer Agents for Conventional Free Radical Linear and Branched Vinyl Polymerization. J. Polym. Sci. A Polym. Chem. 2017, 55, 3963–3967. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cong, H.; Li, L.; Zheng, S. Thermoresponse Improvement of Poly(N-Isopropylacrylamide) Hydrogels Via Formation of Poly(Sodium P-Styrenesulfonate) Nanophases. ACS Appl. Mater. Interfaces 2014, 6, 13677–13687. [Google Scholar] [CrossRef] [PubMed]
- Kjøniksen, A.L.; Zhu, K.; Pamies, R.; Nyström, B. Temperature-Induced Formation and Contraction of Micelle-Like Aggregates in Aqueous Solutions of Thermoresponsive Short-Chain Copolymers. J. Phys. Chem. B 2008, 112, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Kjøniksen, A.-L.; Zhu, K.; Karlsson, G.; Nyström, B. Novel Transition Behavior in Aqueous Solutions of A Charged Thermoresponsive Triblock Copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 32–45. [Google Scholar] [CrossRef]
- McFaul, C.A.; Alb, A.M.; Drenski, M.F.; Reed, W.F. Simultaneous Multiple Sample Light Scattering Detection of LCST during Copolymer Synthesis. Polymer 2011, 52, 4825–4833. [Google Scholar] [CrossRef]
- Behrens, M.A.; Kjøniksen, A.-L.; Zhu, K.; Nyström, B.; Pedersen, J.S. Small-Angle X-Ray Scattering Study of Charged Triblock Copolymers as a Function of Polymer Concentration, Temperature, and Charge Screening. Macromolecules 2011, 45, 246–255. [Google Scholar] [CrossRef]
- Takeoka, H.; Wada, S.; Yusa, S.-I.; Sakurai, S.; Nakamura, Y.; Fujii, S. Thermo-Responsive Polypyrrole-Palladium NanocompositeParticles Synthesized by Aqueous Chemical Oxidative Dispersion Polymerization. J. Adhes. Soc. Jpn. 2015, 51, 255–263. [Google Scholar] [CrossRef]
- Mizusaki, M.; Endo, T.; Nakahata, R.; Morishima, Y.; Yusa, S.-I. pH-Induced Association and Dissociation of Intermolecular Complexes Formed by Hydrogen Bonding between Diblock Copolymers. Polymers 2017, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Farnham, W.B.; Moad, G.; Thang, S.H.; Rizzardo, E.; Fryd, M. (CSIRO), Method for Removing Sulfur-Containing End Groups. WO2005113612A1, 12 May 2004. [Google Scholar]
- Uğuzdoğan, E.; Denkbaş, E.B.; Kabasakal, O.S. Investigation of Temperature Sensitivity Behaviors of Water Soluble Polyacrylamides. J. Appl. Polym. Sci. 2013, 127, 4374–4384. [Google Scholar] [CrossRef]
- Masci, G.; Giacomelli, L.; Crescenzi, V. Atom Transfer Radical Polymerization of Sodium2-Acrylamido-2-Methylpropanesulfonate. J. Polym. Sci. A Polym. Chem. 2005, 43, 4446–4454. [Google Scholar] [CrossRef]
- Yusa, S.-i.; Shimada, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. Heat-Induced Association and Dissociation Behavior of Amphiphilic Diblock Copolymers Synthesized via Reversible Addition-Fragmentation Chain Transfer Radical Polymerization. Macromolecules 2004, 37, 7507–7513. [Google Scholar] [CrossRef]
- Masci, G.; Diociaiuti, M.; Crescenzi, V. ATRP Synthesis and Association Properties of Thermoresponsive Anionic Block Copolymers. J. Polym. Sci. A Polym. Chem. 2008, 46, 4830–4842. [Google Scholar] [CrossRef]
- Yamazaki, M.; Akiyama, E.; Ozoe, S. Synthesis of Poly(styrenesulfonate)-poly(dialkylacrylamide) Block Copolymers and Preparation of Conductive PEDOT Composites. Kobunshi Ronbunshu 2017, 74, 524–533. [Google Scholar] [CrossRef]
- Liu, X.; Rao, P.; Xiao, W.; Xiao, Q.; Zhang, W. Synthesis And Performance of Fluid Loss Agents Based On Different Acrylamide Monomers. J. Petro. Explor. Prod. Technol. 2015, 5, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, D.; Yasuda, A.; Osakada, K. Pd Complex-Catalyzed Copolymerization of A Bicyclic Methylenecyclopropane With Carbon Monoxide to Afford A New Polyketone. Dalton Trans. 2003, 2029–2035. [Google Scholar] [CrossRef]
- Takeuchi, D.; Okada, T.; Kuwabara, J.; Osakada, K. Living Alternating Copolymerization of a Methylenecyclopropane Derivative with CO to Afford Polyketone with Dihydrophenanthrene-1,10-diyl Groups. Macromol. Chem. Phys. 2006, 207, 1546–1555. [Google Scholar] [CrossRef]
- Sjoegren, M.; Hansson, S.; Norrby, P.O.; Aakermark, B.; Cucciolito, M.E.; Vitagliano, A. Selective Stabilization of The Anti Isomer of (η3-Allyl)Palladium And -Platinum Complexes. Organometallics 1992, 11, 3954–3964. [Google Scholar] [CrossRef]
- Burger, P.; Baumeister, J. Transition Metal Complexes With Sterically Demanding Ligands. I. Synthesis And X-Ray Crystal Structure of 1,5-Cyclooctadiene Palladium Methyl Triflate, (COD)Pd(Me)(Otf) And Its Cationic Penta-Coordinate Adducts with Sterically Demanding 2,9-Diaryl-Substituted 1,10-Phenanthroline Ligands. J. Organomet. Chem. 1999, 575, 214–222. [Google Scholar] [CrossRef]
- Uozumi, Y.; Purta, A.E.; Ichii, S.; Tazawa, A. C-H Arylation of Thiophenes with Aryl Bromides by a Parts-per-Million Loading of a Palladium NNC-Pincer Complex. Synlett 2020, 31, 1634–1638. [Google Scholar] [CrossRef]
- Ichii, S.; Hamasaka, G.; Uozumi, Y. The Hiyama Cross-Coupling Reaction at Parts Per Million Levels of Pd: In Situ Formation of Highly Active Spirosilicates in Glycol Solvents. Chem. Asian J. 2019, 14, 3850–3854. [Google Scholar] [CrossRef]
- Hamasaka, G.; Sakurai, F.; Uozumi, Y. A Palladium NNC-Pincer Complex: An Efficient Catalyst for Allylic Arylation at Parts Per Billion Levels. Chem. Commun. 2015, 51, 3886–3888. [Google Scholar] [CrossRef]
- Nguefack, J.-F.; Bolitt, V.; Sinou, D. An Efficient Palladium-Catalysed Coupling of Terminal Alkynes with Aryl Halides under Jeffery’s Conditions. Tetrahedron Lett. 1996, 37, 5527–5530. [Google Scholar] [CrossRef]
- Alami, M.; Ferri, F.; Linstrumelle, G. An Efficient Palladium-Catalysed Reaction of Vinyl And Aryl Halides or Triflates with Terminal Alkynes. Tetrahedron Lett. 1993, 34, 6403–6406. [Google Scholar] [CrossRef]
- Uozumi, Y.; Kobayashi, Y. The Sonogashira Reaction in Water via an Amphiphilic Resin-supported Palladium-Phosphine Complex under Copper-free Conditions. Heterocycles 2003, 59, 71–74. [Google Scholar] [CrossRef]
- Liang, B.; Dai, M.; Chen, J.; Yang, Z. Copper-Free Sonogashira Coupling Reaction with PdCl2 in Water under Aerobic Conditions. J. Org. Chem. 2005, 70, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Anderson, K.W.; Zim, D.; Jiang, L.; Klapars, A.; Buchwald, S.L. Expanding Pd-Catalyzed C-N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions. J. Am. Chem. Soc. 2003, 125, 6653–6655. [Google Scholar] [CrossRef]
- Handa, S.; Jin, B.; Bora, P.P.; Wang, Y.; Zhang, X.; Gallou, F.; Reilly, J.; Lipshutz, B.H. Sonogashira Couplings Catalyzed by Fe Nanoparticles Containing ppm Levels of Reusable Pd, under Mild Aqueous Micellar Conditions. ACS Catal. 2019, 9, 2423–2431. [Google Scholar] [CrossRef]
- Komaromi, A.; Novak, Z. Efficient Copper-Free Sonogashira Coupling of Aryl Chlorides with Palladium on Charcoal. Chem. Commun. 2008, 4968–4970. [Google Scholar] [CrossRef]







| Entry | PdCl2(PPh3)2/mol% | Copolymer (Surfactant) | Yield of 5aa/% b |
|---|---|---|---|
| 1 | 2 | NS | 99 |
| 2 | 0.5 | NS | 52 |
| 3 | 2 | NA | 73 |
| 4 | 2 | DS | 99 |
| 5 | 0.5 | DS | 49 |
| 6 | 2 | DA | 99 |
| 7 | 2 | SDS | 47 |
| 8 | 2 | Triton X-100 | 61 |
| 9 c | 2 | none | 43 |

| Entry | 1/mol% b | H2NNH2/mol% b | Yield/% | TON |
|---|---|---|---|---|
| 1 | 0.5 | 0 | 46 | 92 |
| 2 | 0.5 | 6.5 | 97 | 194 |
| 3 c | 0.1 | 0.65 | 99 | 990 |
| 4 | 0.01 | 0.065 | 64 | 6400 |
| 5 d | 0.01 | 0.065 | 78 | 7800 |

| Entry | Aryl halide | Alkene | Copolymer | Product | Yield/% a |
|---|---|---|---|---|---|
| 1 |  |  | NS |  | >99 b |
| 2 |  |  | NA |  | 15 b |
| 3 |  |  | DS |  | 93 b |
| 4 |  |  | DA |  | 98 b |
| 5 |  |  | NS |  | 8 b |
| 6 |  |  | NS |  | 97 (91) |
| 7 |  |  | NS |  | 99 |
| 8 |  |  | NS |  | 98 (85) |
| 9 |  |  | NS |  | 98 |
| 10 |  |  | NS |  | 99 (87) |
| 11 |  |  | NS |  | 89 (83) |
| 12 |  |  | NS |  | 99 (90) |
| 13 |  |  | NS |  | 20 (19) |
| 14 |  |  | NS |  | 13 |
| 15 |  |  | NS |  | 4 |
| 16 |  |  | NS |  | N.R. |
| 17 |  |  | NS |  | N.R. |
| 18 |  |  | NS |  | 6 |
| 19 |  |  | NS |  | trace |
| Entry | Surfactant | Description | Recovery of 5aa/% b | |
|---|---|---|---|---|
| With Diethyl Ether | With Ethyl Acetate | |||
| 1 c | None | Only water | 53(±4) | 61(±8) |
| 2 | NS | PNIPAAm-b-PSSNa | 25 | 40(±23) |
| 3 | NA | PNIPAAm-b-PAMPSNa | – | 69 |
| 4 | DS | PDEAAm-b-PSSNa | 61 | 72 |
| 5 | DA | PDEAAm-b-PAMPSNa | 69 | 76 |
| 6 | SDS | anionic surfactant | 33 | 75 |
| 7 d | DA | PDEAAm-b-PAMPSNa | – | 83 |
| 8 e | DA | PDEAAm-b-PAMPSNa | – | 68 |

| Entry | Catalysts | x/mol % | Additives | y/mol % | Yields/% b |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | 2 | - | - | 38 |
| 2 | PdCl2(PPh3)2 | 2 | - | - | 86 |
| 3 | PdCl2(PPh3)2 | 1 | - | - | 81 |
| 4 | PdCl2(dppf) | 2 | - | - | 63 |
| 5 | PdCl2(dppf) | 1 | - | - | 62 |
| 6 | Pd(OAc)2 | 2 | PPh3 | 2 | 50 |
| 7 | Pd(OAc)2 | 1 | PCy3 | 2 | 14 |
| 8 | Pd(OAc)2 | 2 | XPhos | 4 | 99 |
| 9 | PdCl2(cod) | 2 | XPhos | 4 | 97 |
| 10 | 1 | 0.5 | H2NNH2 | 10 | 74 |
| 11 | PdCl2(PPh3)2 | 2 | CuI | 2 | 99 |
| 12 | Ni(acac)2 | 2 | XPhos | 4 | 7 |
| 13 | NiCl2(PPh3)2 | 2 | - | - | n.r. |

| Entry | Surfactant | Description | Yield of 7da/% b |
|---|---|---|---|
| 1 | None | Only water | 77 |
| 2 | NS | PNIPAAm-b-PSSNa | 86 |
| 3 | NA | PNIPAAm-b-PAMPSNa | 84 |
| 4 | DS | PDEAAm-b-PSSNa | 81 |
| 5 | DA | PDEAAm-b-PAMPSNa | 82 |
| 6 | SDS | Anionic surfactant | 61 |
| 7 | Triton X-100 | Neutral surfactant | 78 |

| Entry | Ar-X | R | Pd | Product | Yield/% b |
|---|---|---|---|---|---|
| 1 |  | Ph 6a | PdCl2(PPh3)2 |  | 86 |
| 2 |  | Ph 6a | PdCl2(PPh3)2 | 7da | 14 |
| 3 |  | Ph 6a | PdCl2(PPh3)2 |  | 77 |
| 4 |  | Ph 6a | PdCl2(PPh3)2 |  | 78 |
| 5 |  | Ph 6a | PdCl2(PPh3)2 |  | 49 c |
| 6 |  | Ph 6a | Pd(OAc)2 + 2 XPhos | 7da | 97 |
| 7 |  | Ph 6a | Pd(OAc)2 + 2 XPhos | 7da | trace |
| 8 |  | Ph 6a | Pd(OAc)2 + 2 XPhos |  | 94 |
| 9 |  | Ph 6a | Pd(OAc)2 + 2 XPhos |  | 84 |
| 10 |  | SiMe2(t-Bu) 6b | Pd(OAc)2 + 2 XPhos |  | 90 |
| 11 |  | SiMe2(t-Bu) 6b | Pd(OAc)2 + 2 XPhos |  | 33 |
| 12 |  | n-C6H13 6c | Pd(OAc)2 + 2 XPhos |  | 94 |
| 13 |  | Ph 6a | Pd(OAc)2 + 2 XPhos |  | 78 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, N.; Koyama, S.; Koike, R.; Ebara, N.; Arai, R.; Takeoka, Y.; Rikukawa, M.; Tsai, F.-Y. Palladium-Catalyzed Mizoroki–Heck and Copper-Free Sonogashira Coupling Reactions in Water Using Thermoresponsive Polymer Micelles. Polymers 2021, 13, 2717. https://doi.org/10.3390/polym13162717
Suzuki N, Koyama S, Koike R, Ebara N, Arai R, Takeoka Y, Rikukawa M, Tsai F-Y. Palladium-Catalyzed Mizoroki–Heck and Copper-Free Sonogashira Coupling Reactions in Water Using Thermoresponsive Polymer Micelles. Polymers. 2021; 13(16):2717. https://doi.org/10.3390/polym13162717
Chicago/Turabian StyleSuzuki, Noriyuki, Shun Koyama, Rina Koike, Nozomu Ebara, Rikito Arai, Yuko Takeoka, Masahiro Rikukawa, and Fu-Yu Tsai. 2021. "Palladium-Catalyzed Mizoroki–Heck and Copper-Free Sonogashira Coupling Reactions in Water Using Thermoresponsive Polymer Micelles" Polymers 13, no. 16: 2717. https://doi.org/10.3390/polym13162717
APA StyleSuzuki, N., Koyama, S., Koike, R., Ebara, N., Arai, R., Takeoka, Y., Rikukawa, M., & Tsai, F. -Y. (2021). Palladium-Catalyzed Mizoroki–Heck and Copper-Free Sonogashira Coupling Reactions in Water Using Thermoresponsive Polymer Micelles. Polymers, 13(16), 2717. https://doi.org/10.3390/polym13162717