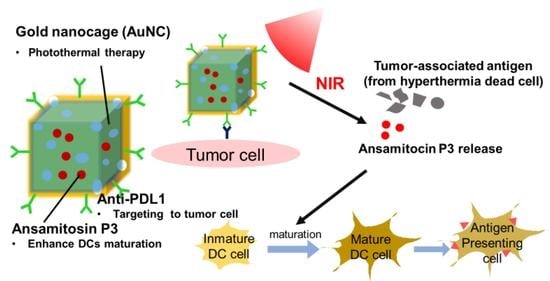
Ansamitocin P3-Loaded Gold-NanoCage Conjugated with Immune Checkpoint Inhibitor to Enhance Photo-Chemo-Thermal Maturation of Dendritic Cells for Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Gold Nanocages (Auncs)
2.2.2. Synthesis of Cys-Biotin-Avidin-Biotin-Anti-PDL1
2.2.3. Synthesis of AP3-Auncs-Cys-Biotin-Avidin-Biotin-Anti-PDL1 (AP3-AuNCs-anti-PDL1)
2.2.4. Characterization of AP3-Auncs-Anti-PDL1
2.2.5. Photothermic Effect of Aqueous AP3-Auncs
2.2.6. Drug-Loading Ability and in Vitro Drug Release
2.2.7. Cell Culture
2.2.8. Determination of Targeting Efficiency of AP3-Auncs-Anti-PDL1
2.2.9. Cytotoxicity Assay of Hep55.1c Treated with Photothermal Therapy
2.2.10. Analysis of Mature Dcs Surface-Marker Expression
3. Result and Discussion
3.1. Synthesis of Silver Nanocubes (Agncs) and Gold Nanocages (Auncs)
3.2. Characterization of Ansamitocin P3-Loaded Gold Nanocages (AP3-Auncs)
3.3. Heating Efficiency and AP3 Release of AP3-Auncs
3.4. Characteristic of Anti-PDL1-Targeted AP3-Auncs
3.5. Cellular Targeting Efficiency of AP3-Auncs-Anti-PDL1
3.6. Cytotoxicity Assay and Photothermal Therapy of AP3-Auncs
3.7. In Vitro Dcs Maturation Efficiency of AP3-Auncs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Iamele, L.; Vecchia, L. Antibody–Drug conjugates: Targeted weapons against cancer. Antib. Technol. J. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Johnston, M.P.; Khakoo, S.I. Immunotherapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. 2019, 25, 2977–2989. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Keenan, B.P.; Fong, L.; Kelley, R.K. Immunotherapy in hepatocellular carcinoma: The complex interface between inflammation, fibrosis, and the immune response. J. Immunother. Cancer 2019, 7, 267. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Fiering, S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int. J. Hyperth. 2014, 30, 531–539. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-Thermal ablations—Boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, S.; Wang, Y.; He, X.; Zhang, Q.; Qi, Y.; Zhou, D.; Xie, Z.; Li, X.; Huang, Y. Synergistic enhancement of immunological responses triggered by hyperthermia sensitive Pt NPs via NIR laser to inhibit cancer relapse and metastasis. Bioact. Mater. 2021. [Google Scholar] [CrossRef]
- Liu, Q.; Zhai, B.; Yang, W.; Yu, L.-X.; Dong, W.; He, Y.-Q.; Chen, L.; Tang, L.; Lin, Y.; Huang, D.-D.; et al. Abrogation of Local Cancer Recurrence After Radiofrequency Ablation by Dendritic Cell-based Hyper-thermic Tumor Vaccine. Mol. Ther. 2009, 17, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liang, F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Park, H.-B.; Kwak, M.; Oh, J.; Lee, P.C.W.; Jin, J.-O. Indocyanine green and poly I:C containing ther-mo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer 2019, 7, 220. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, K.; Zhao, R.; Ji, T.; Wang, X.; Yang, X.; Zhang, Y.; Cheng, K.; Liu, S.; Hao, J.; et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomater. 2016, 102, 187–197. [Google Scholar] [CrossRef]
- Martin, K.; Müller, P.; Schreiner, J.; Prince, S.S.; Lardinois, D.; Heinzelmann-Schwarz, V.; Thommen, D.S.; Zippelius, A. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol. Immunother. 2014, 63, 925–938. [Google Scholar] [CrossRef]
- Qian, X.; Peng, X.-H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2007, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, X.; Kim, C.; Sun, G.; Zhang, Y.S.; Deng, R.; Yang, M.; Chen, J.; Achilefu, S.; Wang, L.; et al. Gold nanocages covered with thermally-responsive polymers for controlled release by high-intensity focused ultrasound. Nanoscale 2011, 3, 1724–1730. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wei, Q.; Wang, S.; Li, F.; Wu, J.; Ren, J.; Cao, F.; Liao, H.; Gao, J.-Q.; Zhou, M.; et al. Molecular engineering of D–A–D conjugated small molecule nanoparticles for high performance NIR-II photothermal therapy. Mater. Horizons 2020, 7, 1379–1386. [Google Scholar] [CrossRef]
- Iwamoto, N.; Shimomura, A.; Tamura, K.; Hamada, A.; Shimada, T. LC–MS bioanalysis of trastuzumab and released em-tansine using nano-surface and molecular-orientation limited (nSMOL) proteolysis and liquid–liquid partition in plasma of trastuzumab emtansine-treated breast cancer patients. J. Pharm. Biomed. Anal. 2017, 145, 33–39. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jain, S.K.; Jaggi, M. A stepwise procedure for isolation of murine bone marrow and gener-ation of dendritic cells. J. Biol. Methods 2014, 1, e1. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Stability and Agglomeration of Alumina Nanoparticles in Ethanol-Water Mixtures. Procedia Eng. 2016, 148, 290–297. [Google Scholar] [CrossRef]
- Tilaki, R.M.; Zad, A.I.; Mahdavi, S.M. The effect of liquid environment on size and aggregation of gold nanoparticles prepared by pulsed laser ablation. J. Nanoparticle Res. 2006, 9, 853–860. [Google Scholar] [CrossRef]
- Nikolić, V.D.; Ilić, D.P.; Nikolić, L.B.; Stanković, M.Z.; Stanojević, L.P.; Savić, I.M.; Savić, I.M. The synthesis and structure char-acterization of deoxyalliin and alliin. Adv. Technol. 2012, 1, 38–46. [Google Scholar]
- Shan, B.; Wang, H.; Li, L.; Zhou, G.; Wen, Y.; Chen, M.; Li, M. Rationally designed dual-plasmonic gold nanorod@cuprous sele-nide hybrid heterostructures by regioselective overgrowth for in vivo photothermal tumor ablation in the second near-infrared biowindow. Theranostics 2020, 10, 11656–11672. [Google Scholar] [CrossRef]
- Wang, J.; Mei, T.; Liu, Y.; Zhang, Y.; Zhang, Z.; Hu, Y.; Wang, Y.; Wu, M.; Yang, C.; Zhong, X.; et al. Dual-targeted and MRI-guided photothermal therapy via iron-based nanoparticles-incorporated neutrophils. Biomater. Sci. 2021, 9, 3968–3978. [Google Scholar] [CrossRef]
- Chiang, C.-S.; Lin, Y.-J.; Lee, R.; Lai, Y.-H.; Cheng, H.-W.; Hsieh, C.-H.; Shyu, W.-C.; Chen, S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef]
- Dougan, M.; Ingram, J.R.; Jeong, H.-J.; Mosaheb, M.M.; Bruck, P.T.; Ali, L.; Pishesha, N.; Blomberg, O.; Tyler, P.M.; Servos, M.M.; et al. Targeting Cytokine Therapy to the Pancreatic Tumor Microenvironment Using PD-L1–Specific VHHs. Cancer Immunol. Res. 2018, 6, 389–401. [Google Scholar] [CrossRef]
- Calderaro, J.; Rousseau, B.; Amaddeo, G.; Mercey, M.; Charpy, C.; Costentin, C.; Luciani, A.; Zafrani, E.-S.; Laurent, A.; Azoulay, D.; et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship With clinical and pathological features. Hepatology 2016, 64, 2038–2046. [Google Scholar] [CrossRef]
- Kudo, M. Immuno-Oncology in Hepatocellular Carcinoma: 2017 Update. Oncol. 2017, 93, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, J.; Lu, K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem. Biophys. Res. Commun. 2017, 486, 239–244. [Google Scholar] [CrossRef]
- Yang, S.; Yao, D.; Wang, Y.; Yang, W.; Zhang, B.; Wang, D. Enzyme-triggered self-assembly of gold nanoparticles for enhanced retention effects and photothermal therapy of prostate cancer. Chem. Commun. 2018, 54, 9841–9844. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Q.; Chen, J.; Liu, L.; Ding, L.; Shen, M.; Li, J.; Han, B.; Duan, Y. Temperature-Sensitive Gold Nanoparticle-Coated Pluronic-PLL Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy. Theranostics 2017, 7, 4424–4444. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Zhang, H.; Yu, J.; Tong, S.; Tian, N.; Wang, Z.; Wang, X.; Su, X.; Chu, X.; Lin, J.; et al. Monodis-Perse Au–Fe2C Janus Nanoparticles: An Attractive Multifunctional Material for Triple-Modal Imaging-Guided Tumor Pho-tothermal Therapy. ACS Nano 2017, 11, 9239–9248. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-W.; Ou, Y.-L.; Kuo, C.-C.; Tsao, H.-Y.; Lu, H.-E. Ansamitocin P3-Loaded Gold-NanoCage Conjugated with Immune Checkpoint Inhibitor to Enhance Photo-Chemo-Thermal Maturation of Dendritic Cells for Hepatocellular Carcinoma. Polymers 2021, 13, 2726. https://doi.org/10.3390/polym13162726
Cheng H-W, Ou Y-L, Kuo C-C, Tsao H-Y, Lu H-E. Ansamitocin P3-Loaded Gold-NanoCage Conjugated with Immune Checkpoint Inhibitor to Enhance Photo-Chemo-Thermal Maturation of Dendritic Cells for Hepatocellular Carcinoma. Polymers. 2021; 13(16):2726. https://doi.org/10.3390/polym13162726
Chicago/Turabian StyleCheng, Hung-Wei, Yu-Ling Ou, Chia-Chi Kuo, Hsin-Yi Tsao, and Huai-En Lu. 2021. "Ansamitocin P3-Loaded Gold-NanoCage Conjugated with Immune Checkpoint Inhibitor to Enhance Photo-Chemo-Thermal Maturation of Dendritic Cells for Hepatocellular Carcinoma" Polymers 13, no. 16: 2726. https://doi.org/10.3390/polym13162726
APA StyleCheng, H. -W., Ou, Y. -L., Kuo, C. -C., Tsao, H. -Y., & Lu, H. -E. (2021). Ansamitocin P3-Loaded Gold-NanoCage Conjugated with Immune Checkpoint Inhibitor to Enhance Photo-Chemo-Thermal Maturation of Dendritic Cells for Hepatocellular Carcinoma. Polymers, 13(16), 2726. https://doi.org/10.3390/polym13162726